NEET-XII-Chemistry
08: The d-and f-Block Elements
- #15Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
() iodide () iron(II) solution and () H2S
() iron(II) solution and () H2S
() iron(II) solution and () H2S
() iron(II) solution and () H2SAns : acts as a very strong oxidising agent in the acidic medium.
acts as a very strong oxidising agent in the acidic medium.
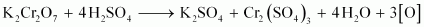
 takes up electrons to get reduced and acts as an oxidising agent. The reaction of K2Cr2O7 with other iodide, iron (II) solution, and H2S are given below.
takes up electrons to get reduced and acts as an oxidising agent. The reaction of K2Cr2O7 with other iodide, iron (II) solution, and H2S are given below.
() oxidizes iodide to iodine.
oxidizes iodide to iodine.
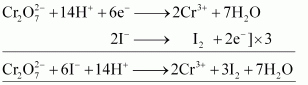
() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

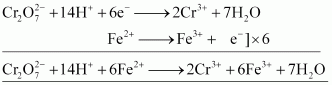
() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

- #15-iiodide () iron(II) solution and () H2S
() iron(II) solution and () H2S
() iron(II) solution and () H2SAns : oxidizes iodide to iodine.
oxidizes iodide to iodine.
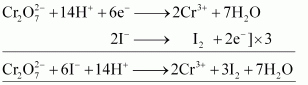
() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

() oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

() oxidizes H2S to sulphur.
oxidizes H2S to sulphur.

- #15-iiiron(II) solution andAns :
 oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.
oxidizes iron (II) solution to iron (III) solution i.e., ferrous ions to ferric ions.

- #15-iiiH2SAns :
 oxidizes H2S to sulphur.
oxidizes H2S to sulphur.
