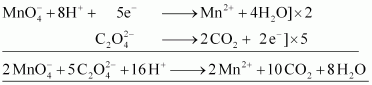NEET-XII-Chemistry
08: The d-and f-Block Elements
- #16Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with () iron(II) ions () SO2 and () oxalic acid?
Write the ionic equations for the reactions.
Ans : Potassium permanganate can be prepared from pyrolusite (MnO2). The ore is fused with KOH in the presence of either atmospheric oxygen or an oxidising agent, such as KNO3 or KClO4, to give K2MnO4.
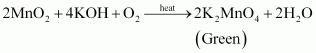
The green mass can be extracted with water and then oxidized either electrolytically or by passing chlorine/ozone into the solution.
Electrolytic oxidation
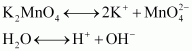
At anode, manganate ions are oxidized to permanganate ions.
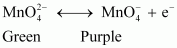
Oxidation by chlorine
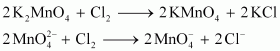
Oxidation by ozone
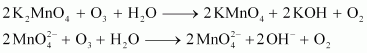
() Acidified KMnO4 solution oxidizes Fe (II) ions to Fe (III) ions i.e., ferrous ions to ferric ions.

() Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
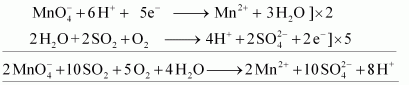
() Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
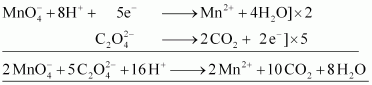
- #16-iiron(II) ionsAns : Acidified KMnO4 solution oxidizes Fe (II) ions to Fe (III) ions i.e., ferrous ions to ferric ions.

- #16-iiSO2 andAns : Acidified potassium permanganate oxidizes SO2 to sulphuric acid.
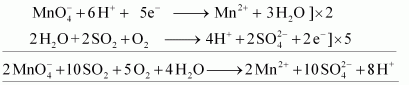
- #16-iiioxalic acid?
Write the ionic equations for the reactions.
Ans : Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.