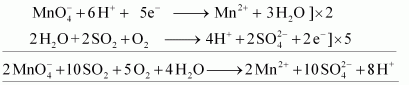NEET-XII-Chemistry
08: The d-and f-Block Elements
- Qstn #16-iiioxalic acid?
Write the ionic equations for the reactions.
Ans : Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
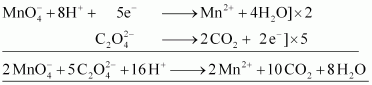
- Qstn #17For M2+/M and M3+/M2+ systems, the
 values for some metals are as follows:
values for some metals are as follows:
Cr2+/Cr -0.9V
Cr3/Cr2+ -0.4 V
Mn2+/Mn -1.2V
Mn3+ /Mn2+ +1.5 V
Fe2+ /Fe -0.4V
Fe3+/Fe2+ +0.8 V
Use this data to comment upon:
- Qstn #17-iThe stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+ andAns : The
 value for Fe3+/Fe2+ is higher than that for Cr3+/Cr2+ and lower than that for Mn3+/Mn2+. So, the reduction of Fe3+ to Fe2+ is easier than the reduction of Mn3+ to Mn2+, but not as easy as the reduction of Cr3+ to Cr2+. Hence, Fe3+ is more stable than Mn3+, but less stable than Cr3+. These metal ions can be arranged in the increasing order of their stability as: Mn3+ < Fe3+ < Cr3+
value for Fe3+/Fe2+ is higher than that for Cr3+/Cr2+ and lower than that for Mn3+/Mn2+. So, the reduction of Fe3+ to Fe2+ is easier than the reduction of Mn3+ to Mn2+, but not as easy as the reduction of Cr3+ to Cr2+. Hence, Fe3+ is more stable than Mn3+, but less stable than Cr3+. These metal ions can be arranged in the increasing order of their stability as: Mn3+ < Fe3+ < Cr3+
- Qstn #17-iiThe ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.Ans : The reduction potentials for the given pairs increase in the following order.
Mn2+ / Mn < Cr2+ / Cr < Fe2+ /Fe
So, the oxidation of Fe to Fe2+ is not as easy as the oxidation of Cr to Cr2+ and the oxidation of Mn to Mn2+. Thus, these metals can be arranged in the increasing order of their ability to get oxidised as: Fe < Cr < Mn
- Qstn #18Predict which of the following will be coloured in aqueous solution? Ti3+, V3+,
Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reasons for each.
Ans : Only the ions that have electrons in d-orbital and in which d-d transition is possible will be coloured. The ions in which d-orbitals are empty or completely filled will be colourless as no d-d transition is possible in those configurations.
-
Element
Atomic Number
Ionic State
Electronic configuration in ionic state
Ti
22
T13+
[Ar] 3d1
V
23
V3+
[Ar] 3d2
Cu
29
Cu+
[Ar] 3d10
Sc
21
Sc3+
[Ar]
Mn
25
Mn2+
[Ar] 3d5
Fe
26
Fe3+
[Ar] 3d5
Co
27
Co2+
[Ar] 3d7
From the above table, it can be easily observed that only Sc3+ has an empty d-orbital and Cu+ has completely filled d-orbitals. All other ions, except Sc3+ and Cu+, will be coloured in aqueous solution because of d-d transitions.
-
- Qstn #19Compare the stability of +2 oxidation state for the elements of the first transition series.
Ans :-
Sc +3 Ti +1 +2 +3 +4 V +1 +2 +3 +4 +5 Cr +1 +2 +3 +4 +5 +6 Mn +1 +2 +3 +4 +5 +6 +7 Fe +1 +2 +3 +4 +5 +6 Co +1 +2 +3 +4 +5 Ni +1 +2 +3 +4 Cu +1 +2 +3 Zn +2
From the above table, it is evident that the maximum number of oxidation states is shown by Mn, varying from +2 to +7. The number of oxidation states increases on moving from Sc to Mn. On moving from Mn to Zn, the number of oxidation states decreases due to a decrease in the number of available unpaired electrons. The relative stability of the +2 oxidation state increases on moving from top to bottom. This is because on moving from top to bottom, it becomes more and more difficult to remove the third electron from the d-orbital.
-
- Qstn #20-ielectronic configurationAns : Electronic configuration
The general electronic configuration for lanthanoids is [Xe]54 4f0-14 5d0-1 6s2 and that for actinoids is [Rn]86 5f1-14 6d0-1 7s2. Unlike 4f orbitals, 5f orbitals are not deeply buried and participate in bonding to a greater extent.
- Qstn #20-iiatomic and ionic sizesAns : Oxidation states
The principal oxidation state of lanthanoids is (+3). However, sometimes we also encounter oxidation states of + 2 and + 4. This is because of extra stability of fully-filled and half-filled orbitals. Actinoids exhibit a greater range of oxidation states. This is because the 5f, 6d, and 7s levels are of comparable energies. Again, (+3) is the principal oxidation state for actinoids. Actinoids such as lanthanoids have more compounds in +3 state than in +4 state.
- Qstn #20-iiioxidation stateAns : Atomic and lonic sizes
Similar to lanthanoids, actinoids also exhibit actinoid contraction (overall decrease in atomic and ionic radii). The contraction is greater due to the poor shielding effect of 5f orbitals.
- Chemical reactivity
In the lanthanide series, the earlier members of the series are more reactive. They have reactivity that is comparable to Ca. With an increase in the atomic number, the lanthanides start behaving similar to Al. Actinoids, on the other hand, are highly reactive metals, especially when they are finely divided. When they are added to boiling water, they give a mixture of oxide and hydride. Actinoids combine with most of the non-metals at moderate temperatures. Alkalies have no action on these actinoids. In case of acids, they are slightly affected by nitric acid (because of the formation of a protective oxide layer).
- Qstn #21-iOf the d4species, Cr2+ is strongly reducing while manganese(III) is strongly oxidising.Ans : Cr2+ is strongly reducing in nature. It has a d4 configuration. While acting as a reducing agent, it gets oxidized to Cr3+ (electronic configuration, d3). This d3 configuration can be written as
 configuration, which is a more stable configuration. In the case of Mn3+ (d4), it acts as an oxidizing agent and gets reduced to Mn2+ (d5). This has an exactly half-filled d-orbital and is highly stable.
configuration, which is a more stable configuration. In the case of Mn3+ (d4), it acts as an oxidizing agent and gets reduced to Mn2+ (d5). This has an exactly half-filled d-orbital and is highly stable.
- Qstn #21-iiCobalt(II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.Ans : Co(II) is stable in aqueous solutions. However, in the presence of strong field complexing reagents, it is oxidized to Co (III). Although the 3rd ionization energy for Co is high, but the higher amount of crystal field stabilization energy (CFSE) released in the presence of strong field ligands overcomes this ionization energy.