NEET-XII-Chemistry
08: The d-and f-Block Elements
- #14Describe the preparation of potassium dichromate from iron chromite ore.
What is the effect of increasing pH on a solution of potassium dichromate?
Ans : Potassium dichromate is prepared from chromite ore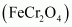 in the following steps.
in the following steps.
Step (1): Preparation of sodium chromate
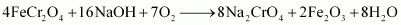
Step (2): Conversion of sodium chromate into sodium dichromate

Step(3): Conversion of sodium dichromate to potassium dichromate

Potassium dichromate being less soluble than sodium chloride is obtained in the form of orange coloured crystals and can be removed by filtration.
The dichromate ion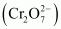 exists in equilibrium with chromate
exists in equilibrium with chromate 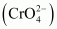 ion at pH 4. However, by changing the pH, they can be interconverted.
ion at pH 4. However, by changing the pH, they can be interconverted.
2CrO42-→Acid2HCrO4-→Acid2Cr2O72-Cr2O72-→Base2HCrO42-→Base2CrO42-