NEET-XII-Chemistry
08: The d-and f-Block Elements
- #8 - The d-and f-Block Elements
- #Section : ISECTION I Page No 212:
- Qstn #1Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
Ans : Ag has a completely filled 4d orbital (4d10 5s1) in its ground state. Now, silver displays two oxidation states (+1 and +2). In the +1 oxidation state, an electron is removed from the s-orboital. However, in the +2 oxidation state, an electron is removed from the d-orbital. Thus, the d-orbital now becomes incomplete (4d9). Hence, it is a transition element.
- Qstn #2In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomization of zinc is the lowest, i.e., 126 kJ mol-1. Why?
Ans : The extent of metallic bonding an element undergoes decides the enthalpy of atomization. The more extensive the metallic bonding of an element, the more will be its enthalpy of atomization. In all transition metals (except Zn, electronic configuration: 3d10 4s2), there are some unpaired electrons that account for their stronger metallic bonding. Due to the absence of these unpaired electrons, the inter-atomic electronic bonding is the weakest in Zn and as a result, it has the least enthalpy of atomization.
- Qstn #3Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
Ans : Mn (Z = 25) = 3d5 4s2
Mn has the maximum number of unpaired electrons present in the d-subshell (5 electrons). Hence, Mn exhibits the largest number of oxidation states, ranging from +2 to +7.
- Qstn #4The Eθ(M2+/M) value for copper is positive (+0.34V). What is possibly the reason for this? (Hint: consider its high ΔaHθ and low ΔhydHθ)
Ans : The Eθ(M2+/M) value of a metal depends on the energy changes involved in the following:
1. Sublimation: The energy required for converting one mole of an atom from the solid state to the gaseous state.
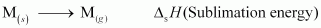
2. Ionization: The energy required to take out electrons from one mole of atoms in the gaseous state.
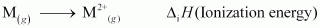
3. Hydration: The energy released when one mole of ions are hydrated.
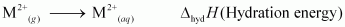
Now, copper has a high energy of atomization and low hydration energy. Hence, the Eθ(M2+/M) value for copper is positive.
- Qstn #5How would you account for the irregular variation of ionization enthalpies (first and second) in the first series of the transition elements?
Ans : Ionization enthalpies are found to increase in the given series due to a continuous filling of the inner d-orbitals. The irregular variations of ionization enthalpies can be attributed to the extra stability of configurations such as d0, d5, d10. Since these states are exceptionally stable, their ionization enthalpies are very high.
In case of first ionization energy, Cr has low ionization energy. This is because after losing one electron, it attains the stable configuration (3d5). On the other hand, Zn has exceptionally high first ionization energy as an electron has to be removed from stable and fully-filled orbitals (3d10 4s2).
Second ionization energies are higher than the first since it becomes difficult to remove an electron when an electron has already been taken out. Also, elements like Cr and Cu have exceptionally high second ionization energies as after losing the first electron, they have attained the stable configuration (Cr+: 3d5 and Cu+: 3d10). Hence, taking out one electron more from this stable configuration will require a lot of energy.
- Qstn #6Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Ans : Both oxide and fluoride ions are highly electronegative and have a very small size. Due to these properties, they are able to oxidize the metal to its highest oxidation state.
- Qstn #7Which is a stronger reducing agent Cr2+ or Fe2+ and why?
Ans : The following reactions are involved when Cr2+ and Fe2+ act as reducing agents.
Cr2+ Cr3+ Fe2+
Cr3+ Fe2+  Fe3+
Fe3+
The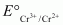 value is -0.41 V and
value is -0.41 V and 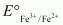 is +0.77 V. This means that Cr2+ can be easily oxidized to Cr3+, but Fe2+ does not get oxidized to Fe3+ easily. Therefore, Cr2+ is a better reducing agent that Fe3+.
is +0.77 V. This means that Cr2+ can be easily oxidized to Cr3+, but Fe2+ does not get oxidized to Fe3+ easily. Therefore, Cr2+ is a better reducing agent that Fe3+.
- Qstn #8Calculate the ‘spin only’ magnetic moment of M2+(aq) ion (Z = 27).
Ans : Z = 27
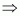 [Ar] 3d7 4s2
[Ar] 3d7 4s2
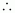 M2+ = [Ar] 3d7
M2+ = [Ar] 3d7
3d7 =
i.e., 3 unpaired electrons
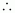 n = 3
n = 3
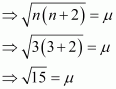
μ ≈ 4 BM
- Qstn #9Explain why Cu+ ion is not stable in aqueous solutions?
Ans : In an aqueous medium, Cu2+ is more stable than Cu+. This is because although energy is required to remove one electron from Cu+ to Cu2+, high hydration energy of Cu2+ compensates for it. Therefore, Cu+ ion in an aqueous solution is unstable. It disproportionates to give Cu2+ and Cu.
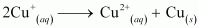
- Qstn #10Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Ans : In actinoids, 5f orbitals are filled. These 5f orbitals have a poorer shielding effect than 4f orbitals (in lanthanoids). Thus, the effective nuclear charge experienced by electrons in valence shells in case of actinoids is much more that that experienced by lanthanoids. Hence, the size contraction in actinoids is greater as compared to that in lanthanoids.
- #Section : II