NEET-XII-Chemistry
08: The d-and f-Block Elements
- Qstn #1-viLu2+Ans : Lu2+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d1
Or, [Xe]54 2f14 3d3
- Qstn #1-viiiTh4+Ans : Th4+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6s6
Or, [Rn]86
- Qstn #2Why are Mn2+compounds more stable than Fe2+ towards oxidation to their +3 state?
Ans : Electronic configuration of Mn2+ is [Ar]18 3d5.
Electronic configuration of Fe2+ is [Ar]18 3d6.
It is known that half-filled and fully-filled orbitals are more stable. Therefore, Mn in (+2) state has a stable d5 configuration. This is the reason Mn2+ shows resistance to oxidation to Mn3+. Also, Fe2+ has 3d6 configuration and by losing one electron, its configuration changes to a more stable 3d5 configuration. Therefore, Fe2+ easily gets oxidized to Fe+3 oxidation state.
- Qstn #3Explain briefly how +2 state becomes more and more stable in the first half
of the first row transition elements with increasing atomic number?
Ans : The oxidation states displayed by the first half of the first row of transition metals are given in the table below.
-
Sc Ti V Cr Mn Oxidation state
+ 2 + 2 + 2 + 2 +3 + 3 + 3 + 3 + 3 + 4 + 4 + 4 + 4 + 5 + 5 + 6 + 6 + 7
It can be easily observed that except Sc, all others metals display +2 oxidation state. Also, on moving from Sc to Mn, the atomic number increases from 21 to 25. This means the number of electrons in the 3d-orbital also increases from 1 to 5.
-
Sc (+2) = d1 Ti (+2) = d2 V (+2) = d3 Cr (+2) = d4 Mn (+2) = d5
+2 oxidation state is attained by the loss of the two 4s electrons by these metals. Since the number of d electrons in (+2) state also increases from Ti(+2) to Mn(+ 2), the stability of +2 state increases (as d-orbital is becoming more and more half-filled). Mn (+2) has d5 electrons (that is half-filled d shell, which is highly stable).
-
- Qstn #4To what extent do the electronic configurations decide the stability of
oxidation states in the first series of the transition elements? Illustrate
your answer with examples.
Ans : The elements in the first-half of the transition series exhibit many oxidation states with Mn exhibiting maximum number of oxidation states (+2 to +7). The stability of +2 oxidation state increases with the increase in atomic number. This happens as more electrons are getting filled in the d-orbital. However, Sc does not show +2 oxidation state. Its electronic configuration is 4s2 3d1. It loses all the three electrons to form Sc3+. +3 oxidation state of Sc is very stable as by losing all three electrons, it attains stable noble gas configuration, [Ar]. Ti (+ 4) and V(+5) are very stable for the same reason. For Mn, +2 oxidation state is very stable as after losing two electrons, its d-orbital is exactly half-filled, [Ar] 3d5.
- Qstn #5What may be the stable oxidation state of the transition element with the
following d electron configurations in the ground state of their atoms : 3d3,
3d5, 3d8and 3d4?
Ans :-
Electronic configuration in ground state
Stable oxidation states
(i) 3d3 (Vanadium) +2, +3, +4 and +5 (ii) 3d5 (Chromium) +3, +4, +6 (iii) 3d5 (Manganese) +2, +4, +6, +7 (iv) 3d8 (Cobalt) +2, +3 (v) 3d4 There is no3d4 configuration in ground state.
-
- Qstn #6Name the oxometal anions of the first series of the transition metals in
which the metal exhibits the oxidation state equal to its group number.
Ans : (i) Vanadate,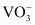
Oxidation state of V is + 5.
(ii) Chromate,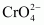
Oxidation state of Cr is + 6.
(iii) Permanganate,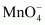
Oxidation state of Mn is + 7.
- Qstn #7What is lanthanoid contraction? What are the consequences of lanthanoid
contraction?
Ans : As we move along the lanthanoid series, the atomic number increases gradually by one. This means that the number of electrons and protons present in an atom also increases by one. As electrons are being added to the same shell, the effective nuclear charge increases. This happens because the increase in nuclear attraction due to the addition of proton is more pronounced than the increase in the interelectronic repulsions due to the addition of electron. Also, with the increase in atomic number, the number of electrons in the 4f orbital also increases. The 4f electrons have poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases. Consequently, the attraction of the nucleus for the outermost electrons increases. This results in a steady decrease in the size of lanthanoids with the increase in the atomic number. This is termed as lanthanoid contraction.
Consequences of lanthanoid contraction
(i) There is similarity in the properties of second and third transition series.
- Separation of lanthanoids is possible due to lanthanide contraction.
(iii) It is due to lanthanide contraction that there is variation in the basic strength of lanthanide hydroxides. (Basic strength decreases from La(OH)3 to Lu(OH)3.)
- Qstn #8What are the characteristics of the transition elements and why are they
called transition elements? Which of the d-block elements may not be
regarded as the transition elements?
Ans : Transition elements are those elements in which the atoms or ions (in stable oxidation state) contain partially filled d-orbital. These elements lie in the d-block and show a transition of properties between s-block and p-block. Therefore, these are called transition elements.
Elements such as Zn, Cd, and Hg cannot be classified as transition elements because these have completely filled d-subshell.
- Qstn #9In what way is the electronic configuration of the transition elements different
from that of the non-transition elements?
Ans : Transition metals have a partially filled d-orbital. Therefore, the electronic configuration of transition elements is (n - 1)d1-10 ns0-2.
The non-transition elements either do not have a d-orbital or have a fully filled d-orbital. Therefore, the electronic configuration of non-transition elements is ns1-2 or ns2 np1-6.