NEET-XII-Chemistry
08: The d-and f-Block Elements
- #4The Eθ(M2+/M) value for copper is positive (+0.34V). What is possibly the reason for this? (Hint: consider its high ΔaHθ and low ΔhydHθ)
Ans : The Eθ(M2+/M) value of a metal depends on the energy changes involved in the following:
1. Sublimation: The energy required for converting one mole of an atom from the solid state to the gaseous state.
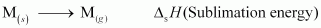
2. Ionization: The energy required to take out electrons from one mole of atoms in the gaseous state.
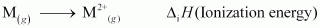
3. Hydration: The energy released when one mole of ions are hydrated.
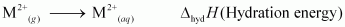
Now, copper has a high energy of atomization and low hydration energy. Hence, the Eθ(M2+/M) value for copper is positive.