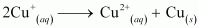NEET-XII-Chemistry
08: The d-and f-Block Elements
- #9Explain why Cu+ ion is not stable in aqueous solutions?
Ans : In an aqueous medium, Cu2+ is more stable than Cu+. This is because although energy is required to remove one electron from Cu+ to Cu2+, high hydration energy of Cu2+ compensates for it. Therefore, Cu+ ion in an aqueous solution is unstable. It disproportionates to give Cu2+ and Cu.