NEET-XII-Chemistry
08: The d-and f-Block Elements
- #7Which is a stronger reducing agent Cr2+ or Fe2+ and why?
Ans : The following reactions are involved when Cr2+ and Fe2+ act as reducing agents.
Cr2+ Cr3+ Fe2+
Cr3+ Fe2+  Fe3+
Fe3+
The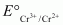 value is -0.41 V and
value is -0.41 V and 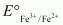 is +0.77 V. This means that Cr2+ can be easily oxidized to Cr3+, but Fe2+ does not get oxidized to Fe3+ easily. Therefore, Cr2+ is a better reducing agent that Fe3+.
is +0.77 V. This means that Cr2+ can be easily oxidized to Cr3+, but Fe2+ does not get oxidized to Fe3+ easily. Therefore, Cr2+ is a better reducing agent that Fe3+.