NEET-XII-Chemistry
Previous Year Paper year:2017
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #16Match the interhalogen compounds of column I
with the geometry in column II and assign the
correct code.
Column I Column II
(a ) XX′ (i) T - shape
(b) XX′3 (ii) Pentagonal bipyramidal
(c) XX′5 (iii) Linear
(d) XX′7 (iv) Square - pyramidal
(v) Tetrahedral
Code :
(a ) (b) (c) (d)
(1) (iv) (iii) (ii) (i)
(2) (iii) (iv) (i) (ii)
(3) (iii) (i) (iv) (ii)
(4) (v) (iv) (iii) (ii)digAnsr: 3Ans : (3)
Sol. (a) XX1 sp3 Linear
(b) 13XX sp
3d T-shape
(c) 15XX sp
3d2 square pyramid
(d) 17XX sp
3d3 Penta genal planar
a ⇒ iii
b ⇒ (i)
c ⇒ (iv)
d ⇒ (ii)
- Qstn #17Mixture of chloroxylenol and terpineol acts as :
(1) antibiotic
(2) analgesic
(3) antiseptic
(4) antipyreticdigAnsr: 3Ans : (3)
Sol. Mixture of Chloroxylenol and terpineol is called dettol which acts as an antiseptic.
- Qstn #18It is because of inability of ``ns^2`` electrons
of the valence shell to participate in bonding that :
(1) ``Sn^{4+}`` is reducing while ``Pb^{4+}`` is oxidising.
(2)``Sn^{2+}`` is reducing while ``Pb^{4+}`` is oxidising.
(3) ``Sn^{2+}`` is oxidising while ``Pb^{4+}`` is reducing.
(4) ``Sn^{2+}`` and ``Pb^{2+}`` are both oxidising and reducing.digAnsr: 2Ans : (2)
Sol. Due to inert pair effect Pb+2 is more stable where as in tin Sn+4 is more stable.
∴ Pb+4 will get reduce and Sn+2 will get Oxidize.
[Pb+4 = oxidising agent, Sn+2 = reducing agent]






- Qstn #19Extraction of gold and silver involves
leaching with ``CN^-`` ion. Silver is later recovered by :
(1) displacement with Zn
(2) liquation
(3) distillation
(4) zone refiningdigAnsr: 1Ans : (1)
Sol. Silver is extracted by cyanide process involving :
1. Complex formation
2. Metal displacement with zinc
Ag2S ⎯⎯ →⎯NaCN Na [Ag(CN)2] + Na2S
Zn
Ag + Na2 [Zn(CN)4]
- Qstn #20A 20 litre container at 400 K contains ``CO_2``(g)
at pressure 0.4 atm and an excess of SrO (neglect the
volume of solid SrO). The volume of the container is now
decreased by moving the movable piston fitted in the
container. The maximum volume of the container, when
pressure of ``CO_2`` attains its maximum value, will be :
(Given that : ``SrCO_3``(s) ``\xrightleftharpoons{K}`` SrO (s) + ``CO_2`` ( g),
``K_p`` = 1.6 atm)
(1) 2 litre
(2) 5 litre
(3) 10 litre
(4) 4 litredigAnsr: 2Ans : (2)
Sol.
)s(
3SrCO )s(
SrO +
)g(
2CO
At maximum pressure of CO2
Kp = 2COP = 1.6 atm
Temperature is constant
∴ P1V1 = P2V2
0.4 × 20 = 1.6 × V2
V2 = 5 lit
- Qstn #21Which is the incorrect statement ?
(1) Frenkel defect is favoured in those ionic compounds
in which sizes of cation and anions are almost equal
(2) FeO0.98 has non stoichiometric metal deficiency defect
(3) Density decreases in case of crystals with Schottky's defect
(4) NaCl(s) is insulator, silicon is semiconductor, silver
is conductor, quartz is piezo electric crystaldigAnsr: 1Ans : (1)
Sol. Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are not equal . i.e.
cation is small in size






- Qstn #22Which of the following is dependent on temperature ?
(1) Weight percentage
(2) Molality
(3) Molarity
(4) Mole fractiondigAnsr: 3Ans : (3)
Sol. Molarity depends upon temperature
VM
1000W
M
A
A
×
×
=
V ∝ T
T ↑ , V ↑,, Molarity (↓)
- Qstn #23The correct order of the stoichiometries of AgCl
formed when AgNO3 in excess is treated with the complexes :
``CoCl_3.6NH_3, CoCl_3.5NH_3, CoCl_3.4NH_3`` respectively is -
(1) 2AgCl, 3AgCl, 1AgCl
(2) 1AgCl, 3AgCl, 2AgCl
(3) 3AgCl, 1AgCl, 2AgCl
(4) 3AgCl, 2AgCl, 1AgCldigAnsr: 4Ans : (4)
Sol. CoCl3.6NH3
[Co(NH3)6]Cl3 ⎯⎯ →⎯ 3AgNO 3AgCl
CoCl3.5NH3
[Co(NH3)5Cl]Cl2 ⎯⎯ →⎯ 3AgNO 2AgCl
CoCl3.4NH3
[Co(NH3)4Cl2]Cl ⎯⎯ →⎯ 3AgNO 1AgCl
- Qstn #24An example of a sigma bonded organometallic compound is -
(1) Cobaltocene
(2) Ruthenocene
(3) Grignard's reagent
(4) FerrocenedigAnsr: 3Ans : (3)
Sol.
Grignard's reagent (R mg x) is σ-bonded organometallic compound CH3-Mg-I
Ferrocene, cobaltocene and Ruthenocene are π-bonded organometallic compound and they contain
cyclopentadionyl ring






- Qstn #25Which one is the wrong statement ?
(1) The energy of 2s orbital is less than the energy
of 2p orbital in case of Hydrogen like atoms
(2) de-Broglie's wavelength is given by λ = ``\frac {h}{mv}``
where m = mass of the particle, v = group velocity of the
particle.
(3) The uncertainty principle is ΔE X Δt ``\geq `` ``\frac{h} {4\pi}``
(4) Half filled and fully filled orbitals have greater
stability due to greater exchange energy, greater
symmetry and more balanced arrangementdigAnsr: 1Ans : (1)
Sol. In hydrogen like atom energy 2s in equal to 2p (as in single electron species)
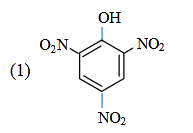
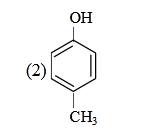
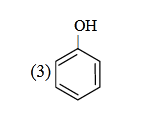
- Qstn #26Which one is the most acidic compound ?
(1)
(2)
(3)
(4) digAnsr: 1Ans : (1)
digAnsr: 1Ans : (1)
Sol. ∵ Acidic strength ∝ stability of anion
|
OH
|
NO2
O2N NO2
|
O-
|
NO2
O2N NO2 -H⊕
Max. stable anion due to delocalised Θve charge & -M effect of all three NO2 group
- Qstn #27A first order reaction has a specific reaction rate
of ``10^{-2} sec^{-1}. How much time will it take for
20 g of the reactant to reduce to 5g ?
(1) 693.0 sec
(2) 238.6 sec
(3) 138.6 sec
(4) 346.5 secdigAnsr: 3Ans : (3)
Sol. For first order reaction

CAREER POINT
CAREER POINT, CP Tower, IPIA, Road No.1, Kota (Raj.), : 0744-6630500 | www.ecareerpoint.com Email: info@cpil.in Page # 11
[ CODE - Y ]
t
0
]A[
]A[log
t
303.2k =
5
20log
t
303.210 2 =-
4log
10
303.2t 2-=
= 64.138
10
6020.0303.2
2 =
×
- sec
- Qstn #28Consider the reactions :
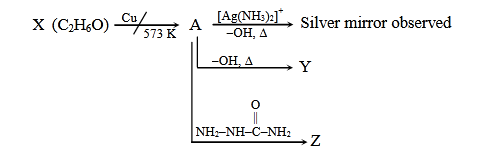
Identify A, X, Y and Z
(1) A-Ethanol, X-Acetaldehyde, Y-Butanone Z-Hydrazone
(2) A-Methoxymethane, X-Ethanoic acid, Y-Acetate ion, Z-Hydrazine
(3) A-Methoxymethane, X-Ethanol, Y-Ethanoic acid, Z-Semicarbazide
(4) A-Ethanal, X-Ethanol, Y-But-2-enal., Z-SemicarbazonedigAnsr: 4Ans : (4)
Sol.
Cu
573 K X
Tollen's
A Y
Z
O
||
NH2-NH-C-NH2
(Oxidation)
Reagent Silver Mirror
OH/Δ
Θ
aldol condensation
semicarbazide
X is CH3CH2-OH (Ethanol)
A is CH3-CH=O (Ethanal)
Y is CH3-CH=CH-CH=O (But-2-enal)
Z is CH3-CH=N-NH-C-NH2
||
O
(Acetaldehyde-semicarbazone)



- Qstn #29Machanism of a hypothetical reaction ``\ce X2+ Y2`` → 2XY is given below.
( i) ``X_ 2`` → X + X (fast)
(ii) X + ``Y_2`` ``\rightleftharpoons`` XY + Y (slow)
(iii) X + Y → XY (fast)
The overall order of the reaction will be -
(1) 1.5
(2) 1
(3) 2
(4) 0digAnsr: 1Ans : (1)
Sol. From slow step
r = k [X] [Y2]
but [X] is dummy reactant so it will replaced by step I
]X[
]X[K
2
2
c =
]X[K]X[ 2c=
∴ 122
1
2
2
1
c ]Y[]X[)K(Kr =
so overall order = 5.11
2
1
=+
- Qstn #30Predict the correct intermediate and product in the
following reaction :

(1)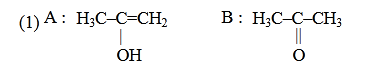
(2)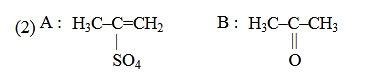
(3)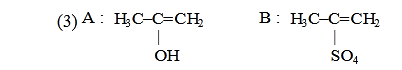
(4) digAnsr: 1Ans : (1)
digAnsr: 1Ans : (1)
Sol.
CH3-C≡CH CH3-C=CH2
OH
H2O
HgSO4
(Markowinikoff
addition)
|
T
CH3-C-CH3
O
||
A B



