NEET-XII-Chemistry
Previous Year Paper year:2017
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #31The IUPAC name of the
compound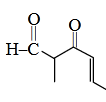 is -
is -
(1) 3-keto-2-methylhex-5-enal
(2) 3-keto-2-methylhex-4-enal
(3) 5-formylhex-2-en-3-one
(4) 5-methyl-4-oxohex-2en-5-aldigAnsr: 2Ans : (2)
Sol.
O
|| CHO
2 3
4
5 6
1
In IUPAC called 3-Keto-2-methyl hex-4-enal
- Qstn #32In the electrochemical cell -
Zn|``ZnSO_4``(0.01M) || ``CuSO_4`` (1.0 M)| Cu,
the emf of this Daniel cell is ``E_1``. When the
concentration of ``ZnSO_4`` is changed to 1.0 M
and that of ``CuSO_4`` changed to 0.01M, the emf
changes to E2. From the followings, which
one is the relationship between E1 and E2 ?
Given, ``\frac {RT}{F}`` = 0.059) .
(1) E2 = 0 ≠ E1
(2) E1 = E2
(3) E1 < E2
(4) E1 > E2digAnsr: 4Ans : (4)
Sol.
Cu||CuSO||ZnSO|Zn
)M0.1(
4
)M01.0(
4
Nernst equation
]Cu[
]Zn[log
2
059.0EEmf 2
2
cell +
+
° -=
In first case
1
01.0log
2
059.0EE cell1 -=
°
In second case
01.0
1log
2
059.0EE cell2 -=
°
So E1 > E2







- Qstn #33A gas is allowed to expand in a well insulated
container against a constant external pressure of
2.5 atm from an initial volume of 2.50 L to a final
volume of 4.50 L. The change in internal energy ΔU
of the gas in joules will be -
(1) +505 J
(2) 1136.25 J
(3) - 500 J
(4) - 505 JdigAnsr: 4Ans : (4)
Sol. ΔU = q + w
Insulated container So, q = 0
ΔU = -PdV
= - 2.5 [4.50 - 2.50]
= - 2.5 × 2 litre - atm = - 5 l atm [1 -atm = 101.3 ≈ 101J]
= - 5 × 101
⇒ - 505 J
- Qstn #34Correct increasing order for the wavelengths
of absorption in the visible region for the
complexes of ``Co^{3+} is -
(1) ``[Co(NH_3)_6]^{3+}, [Co(en)_3]^{3+}, [Co(H_2O)_6]^{3+}``
(2) ``[Co(en)_3]^{3+}, [Co(NH_3)_6]^{3+}, [Co(H_2O)_6]^{3+}``
(3) ``[Co(H_2O)_6]^{3+}, [Co(en)_3]^{3+}, [Co(NH_3)_6]^{3+}``
(4) ``[Co(H_2O)_6]^{3+}, [Co(NH_3)_6]^{3+}, [Co(en)_3]^{3+}``digAnsr: 2Ans : (2)
Sol. Increasing order of wavelength of absorption is
Δ0 = en > NH3 > H2O
λ
==Δ
hcE0
36
3
63
3
3 ])HO(Co[])NH(Co[])en(Co[
+++ <<=λ
- Qstn #35The correct statement regarding electrophile is :
(1) Electrophile can be either neutral or positively
charged species and can form a bond by accepting a
pair of electrons from a nucleophile
(2) Electrophile is a negatively charged species
and can form a bond by accepting a pair of electrons
form a nucleophile
(3) Electrophile is a negatively charged species and
can form a bond by accepting a pair of electrons
from another electrophile
(4) Electrophiles are generally neutral species
and can form a bond by accepting a pair of electrons from a nucleophiledigAnsr: 1Ans : (1)
Sol. Electrophile can be either neutral or positively charged species and can form a bond by accepting a pair of
electrons from a nucleophile.
- Qstn #36For a given reaction, ΔH = 35.5 kJ ``mol^{-1} and
ΔS = 83.6 ``JK^{-1} ``mol^{-1} . The reaction is spontaneous
at : (Assume that ΔH and ΔS do not vary with temperature)
(1) T > 298 K
(2) T < 425 K
(3) T > 425 K
(4) All temperaturesdigAnsr: 3Ans : (3)
Sol. ΔG = ΔH - TΔS
at equilibrium ΔG = 0
35.5 × 103 - T × 83.6 = 0
Teq = 6.83
105.35 3× = 424.64
If T > Teq ; ΔG = -ve
∴ T > 425 K
- Qstn #37Which of the following pairs of compounds is
isoelectronic and isostructural ?
(1)`` IF_3, XeF_2``
(2) ``BeCl_2, XeF_2``
(3) ``TeI_2, XeF_2``
(4) ``IBr_2, XeF_2``digAnsr: 4Ans : (4)
Sol. IBr2- and XeF2 are isoelectronic because they conation same number of valence electron and both are linear.
I Br2 - sp3d linear
Br
Br
I
-
Xe F2 sp3d linear
F
F
Xe
-







- Qstn #38``HgCl_2`` and ``I_2`` both when dissolved in water containing ``I^-``
ions pair of species formed is :
(1) ``Hg_2I_2``, ``I^-``
(2)`` HgI_2``,`` I_3^-``
(3) ``HgI_2`` , ``I^-``
(4)``HgI_4^{2-}`` ,`` I_3^-``digAnsr: 4Ans : (4)
Sol. Due to formation of complex
HgCl2 ⎯→⎯
-I2 HgI2 ⎯→⎯
-I2 [HgI4]-2
I2 ⎯→⎯
-I -3I
- Qstn #39Which one of the following statements is not correct ?
(1) Coenzymes increase the catalytic activity of enzyme
(2) Catalyst does not initiate and reaction
(3) The value of equilibrium constant is changed in the
presence of a catalyst in the reaction at equilibrium
(4) Enzymes catalyse mainly bio-chemical reaction.digAnsr: 3Ans : (3)
Sol. Equilibrium constant does not depend on catalyst.
- Qstn #40Ionic mobility of which of the following alkali
metal ions is lowest when aqueous solutions of their
salts are put under an electric filed?
(1) Li
(2) Na
(3) K
(4) RbdigAnsr: 4Ans : (4)
Sol. According to electrochemistry due to presence of electric field hydration of ions will not place in excess
means it effect will be negligible then only ionic weight is the factor.
Ionic weight of rubidium is high so its mobility will be less.
- Qstn #41The element Z = 114 has been discovered recently.
It will belong to which of the following family/group
and electronic configuration ?
(1) Nitrogen family, [Rn] ``5f^{14}`` ``6d^{10}`` ``7s^2`` ``7p^6``
(2) Halogen family, [Rn] ``5f^{14}`` ``6d^{10}`` ``7s^2`` ``7p^5``
(3) Carbon family, [Rn] ``5f^{14}`` ``6d^{10}`` ``7s^2`` ``7p^2``
(4) Oxygen family, [Rn] ``5f^{14}`` ``6d^{10}`` ``7s^2`` ``7p^4``digAnsr: 3Ans : (3)
Sol. Z = 114
Z114 = [Rn] 5f146d107s27p2
valence configuration is ns2np2 so Z114 belong to carbon family.







CAREER POINT
CAREER POINT, CP Tower, IPIA, Road No.1, Kota (Raj.), : 0744-6630500 | www.ecareerpoint.com Email: info@cpil.in Page # 17
[ CODE - Y ]
- Qstn #42Which one is the correct order of acidity ?
(1) CH3 - CH3 > CH2 = CH2 > CH3 - C ≡ CH > CH≡CH
(2) CH2 = CH2 > CH3 - CH = CH2 > CH3 - C ≡ CH > CH ≡ CH
(3) CH ≡ CH > CH3 - C ≡ CH > CH2 = CH2 > CH3 - CH3
(4) CH ≡ CH > CH2 = CH2 > CH3 - C ≡ CH > CH3 - CH3digAnsr: 3Ans : (3)
Sol. Acidic nature ∝
I
I
+
- ∝ E. N.
∴ Acidic strength order will be
EN(sp carbon) = 3.25 EN(sp2 carbon)
= 2.75
EN(sp3 carbon)
= 2.50
HC ≡ CH > CH3 - C ≡ CH > CH2 = CH2 > CH3 - CH3
- Qstn #43If molality of the dilute solution is doubled,
the value of molal depression constant (``K_f``) will be
(1) unchanged
(2) doubled
(3) halveddigAnsr: 1Ans : (1)
Sol. ΔTf = Kf × m
Kf → does not depend on molality
So, Kf molal depression constant remains same
- Qstn #44The species, having bond angles of 120º is
(1) ``BCl_3``
(2) ``PH_3``
(3) ``CIF_3``
(4) ``NCl_3``digAnsr: 1Ans : (1)
Sol. BCl3 is sp2 hybridized so, BCl3 is trigonal planar and Bond angle is 120º
Cl
B
Cl Cl



- Qstn #45Which of the following reactions is appropriate
for converting acetamide to methanamine ?
(1) Gabriels phthalimide synthesis
(2) Carbylamine reaction
(3) Hoffmann hypobromamide reaction
(4) Stephens reactiondigAnsr: 3Ans : (3)
Sol.
O
CH3 - C - NH2
Br2 + (4) KOH
Or KOBr CH3 - NH2