NEET-XII-Physics
12: Atoms
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- #4 - Atoms
- #1-aThe size of the atom in Thomson’s model is .......... the atomic size in Rutherford’s model. (much greater than/no different from/much less than.)Ans : The sizes of the atoms taken in Thomson’s model and Rutherford’s model have the same order of magnitude.
- #1-bIn the ground state of .......... electrons are in stable equilibrium, while in .......... electrons always experience a net force.
(Thomson’s model/ Rutherford’s model.)
Ans : In the ground state of Thomson’s model, the electrons are in stable equilibrium. However, in Rutherford’s model, the electrons always experience a net force.
- #1-cA classical atom based on .......... is doomed to collapse.
(Thomson’s model/ Rutherford’s model.)
Ans : A classical atom based on Rutherford’s model is doomed to collapse.
- #1-dAn atom has a nearly continuous mass distribution in a .......... but has a highly non-uniform mass distribution in ..........
(Thomson’s model/ Rutherford’s model.)
Ans : An atom has a nearly continuous mass distribution in Thomson’s model, but has a highly non-uniform mass distribution in Rutherford’s model.
- #1-eThe positively charged part of the atom possesses most of the mass in .......... (Rutherford’s model/both the models.)Ans : The positively charged part of the atom possesses most of the mass in both the models.
- Qstn #2Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?
Ans : In the alpha-particle scattering experiment, if a thin sheet of solid hydrogen is used in place of a gold foil, then the scattering angle would not be large enough. This is because the mass of hydrogen (1.67 × 10-27 kg) is less than the mass of incident α-particles (6.64 × 10-27 kg). Thus, the mass of the scattering particle is more than the target nucleus (hydrogen). As a result, the α-particles would not bounce back if solid hydrogen is used in the α-particle scattering experiment.
- Qstn #3What is the shortest wavelength present in the Paschen series of spectral lines?
Ans : Rydberg’s formula is given as:

Where,
h = Planck’s constant = 6.6 × 10-34 Js
c = Speed of light = 3 × 108 m/s
(n1 and n2 are integers)
The shortest wavelength present in the Paschen series of the spectral lines is given for values n1 = 3 and n2 = ∞.
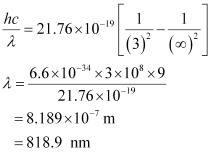
- Qstn #4A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom makes a transition from the upper level to the lower level?
Ans : Separation of two energy levels in an atom,
E = 2.3 eV
= 2.3 × 1.6 × 10-19
= 3.68 × 10-19 J
Let ν be the frequency of radiation emitted when the atom transits from the upper level to the lower level.
We have the relation for energy as:
E = hv
Where,
h = Planck’s constant
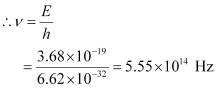
Hence, the frequency of the radiation is 5.6 × 1014 Hz.
- Qstn #5The ground state energy of hydrogen atom is -13.6 eV. What are the kinetic and potential energies of the electron in this state?
Ans : Ground state energy of hydrogen atom, E = - 13.6 eV
This is the total energy of a hydrogen atom. Kinetic energy is equal to the negative of the total energy.
Kinetic energy = - E = - (- 13.6) = 13.6 eV
Potential energy is equal to the negative of two times of kinetic energy.
Potential energy = - 2 × (13.6) = - 27 .2 eV
- Qstn #6A hydrogen atom initially in the ground level absorbs a photon, which excites it to the n = 4 level. Determine the wavelength and frequency of the photon.
Ans : For ground level, n1 = 1
Let E1 be the energy of this level. It is known that E1 is related with n1 as:
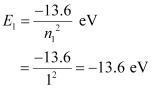
The atom is excited to a higher level, n2 = 4.
Let E2 be the energy of this level.
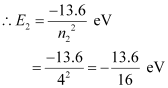
The amount of energy absorbed by the photon is given as:
E = E2 - E1
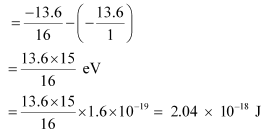
For a photon of wavelengthλ, the expression of energy is written as:

Where,
h = Planck’s constant = 6.6 × 10-34 Js
c = Speed of light = 3 × 108 m/s

And, frequency of a photon is given by the relation,
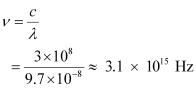
Hence, the wavelength of the photon is 97 nm while the frequency is 3.1 × 1015 Hz.
- #7
- #7-aUsing the Bohr’s model calculate the speed of the electron in a hydrogen atom in the n = 1, 2, and 3 levels.Ans : Let ν1 be the orbital speed of the electron in a hydrogen atom in the ground state level, n1 = 1. For charge (e) of an electron, ν1 is given by the relation,
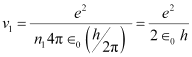
Where,
e = 1.6 × 10-19 C
∈0 = Permittivity of free space = 8.85 × 10-12 N-1 C2 m-2
h = Planck’s constant = 6.62 × 10-34 Js
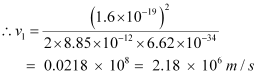
For level n2 = 2, we can write the relation for the corresponding orbital speed as:
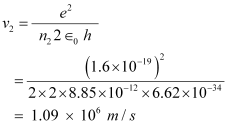
And, for n3 = 3, we can write the relation for the corresponding orbital speed as:
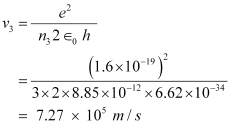
Hence, the speed of the electron in a hydrogen atom in n = 1, n=2, and n=3 is 2.18 × 106 m/s, 1.09 × 106 m/s, 7.27 × 105 m/s respectively.
- #7-bCalculate the orbital period in each of these levels.Ans : Let T1 be the orbital period of the electron when it is in level n1 = 1.
Orbital period is related to orbital speed as:
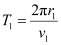
Where,
r1 = Radius of the orbit
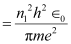
h = Planck’s constant = 6.62 × 10-34 Js
e = Charge on an electron = 1.6 × 10-19 C
∈0 = Permittivity of free space = 8.85 × 10-12 N-1 C2 m-2
m = Mass of an electron = 9.1 × 10-31 kg

For level n2 = 2, we can write the period as:

Where,
r2 = Radius of the electron in n2 = 2


And, for level n3 = 3, we can write the period as:
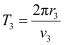
Where,
r3 = Radius of the electron in n3 = 3
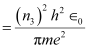
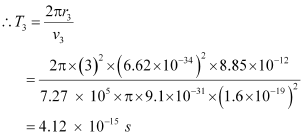
Hence, the orbital period in each of these levels is 1.52 × 10-16 s, 1.22 × 10-15 s, and 4.12 × 10-15 s respectively.