NEET-XII-Physics
12: Atoms
- #4A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom makes a transition from the upper level to the lower level?
Ans : Separation of two energy levels in an atom,
E = 2.3 eV
= 2.3 × 1.6 × 10-19
= 3.68 × 10-19 J
Let ν be the frequency of radiation emitted when the atom transits from the upper level to the lower level.
We have the relation for energy as:
E = hv
Where,
h = Planck’s constant
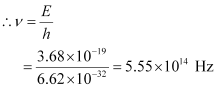
Hence, the frequency of the radiation is 5.6 × 1014 Hz.