NEET-XII-Physics
12: Atoms
- #6A hydrogen atom initially in the ground level absorbs a photon, which excites it to the n = 4 level. Determine the wavelength and frequency of the photon.
Ans : For ground level, n1 = 1
Let E1 be the energy of this level. It is known that E1 is related with n1 as:
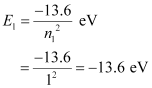
The atom is excited to a higher level, n2 = 4.
Let E2 be the energy of this level.
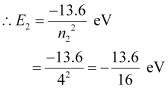
The amount of energy absorbed by the photon is given as:
E = E2 - E1
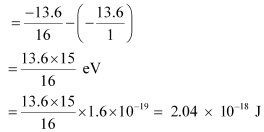
For a photon of wavelengthλ, the expression of energy is written as:

Where,
h = Planck’s constant = 6.6 × 10-34 Js
c = Speed of light = 3 × 108 m/s

And, frequency of a photon is given by the relation,
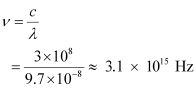
Hence, the wavelength of the photon is 97 nm while the frequency is 3.1 × 1015 Hz.