NEET-XI-Chemistry
06: Chemical Thermodynamics
- #15Calculate the enthalpy change for the process
CCl4(g) → C(g) + 4Cl(g)
and calculate bond enthalpy of C-Cl in CCl4(g).
ΔvapHθ (CCl4) = 30.5 kJ mol-1.
ΔfHθ (CCl4) = -135.5 kJ mol-1.
ΔaHθ (C) = 715.0 kJ mol-1, where ΔaHθ is enthalpy of atomisation
ΔaHθ (Cl2) = 242 kJ mol-1
Ans : The chemical equations implying to the given values of enthalpies are:
 ΔvapHθ = 30.5 kJ mol-1
ΔvapHθ = 30.5 kJ mol-1
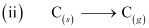 ΔaHθ = 715.0 kJ mol-1
ΔaHθ = 715.0 kJ mol-1
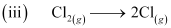 ΔaHθ = 242 kJ mol-1
ΔaHθ = 242 kJ mol-1
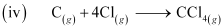 ΔfH = -135.5 kJ mol-1
ΔfH = -135.5 kJ mol-1
Enthalpy change for the given process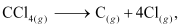 can be calculated using the following algebraic calculations as:
can be calculated using the following algebraic calculations as:
Equation (ii) + 2 × Equation (iii) - Equation (i) - Equation (iv)
ΔH = ΔaHθ(C) + 2ΔaHθ (Cl2) - ΔvapHθ - ΔfH
= (715.0 kJ mol-1) + 2(242 kJ mol-1) - (30.5 kJ mol-1) - (-135.5 kJ mol-1)
.gif) ΔH = 1304 kJ mol-1
ΔH = 1304 kJ mol-1
Bond enthalpy of C-Cl bond in CCl4 (g)
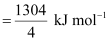
= 326 kJ mol-1