NEET-XI-Chemistry
06: Chemical Thermodynamics
- #17For the reaction at 298 K,
2A + B → C
ΔH = 400 kJ mol-1 and ΔS = 0.2 kJ K-1 mol-1
At what temperature will the reaction become spontaneous considering ΔH and ΔS to be constant over the temperature range?
Ans : From the expression,
ΔG = ΔH - TΔS
Assuming the reaction at equilibrium, ΔT for the reaction would be:
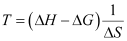
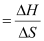 (ΔG = 0 at equilibrium)
(ΔG = 0 at equilibrium)
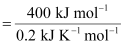
T = 2000 K
For the reaction to be spontaneous, ΔG must be negative. Hence, for the given reaction to be spontaneous, T should be greater than 2000 K.