NEET-XI-Chemistry
06: Chemical Thermodynamics
Qstn# 22 Prvs-Qstn
- #22Calculate the entropy change in surroundings when 1.00 mol of H2O(l) is formed under standard conditions. ΔfHθ = -286 kJ mol-1.
Ans : It is given that 286 kJ mol-1 of heat is evolved on the formation of 1 mol of H2O(l). Thus, an equal amount of heat will be absorbed by the surroundings.
qsurr = +286 kJ mol-1
Entropy change (ΔSsurr) for the surroundings =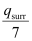

.gif) ΔSsurr = 959.73 J mol-1 K-1
ΔSsurr = 959.73 J mol-1 K-1