NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
Qstn# 40 Prvs-Qstn
- #40What is meant by the term bond order? Calculate the bond order of: N2, O2,
.gif) and
and.gif) .
.
Ans : Bond order is defined as one half of the difference between the number of electrons present in the bonding and anti-bonding orbitals of a molecule.
If Na is equal to the number of electrons in an anti-bonding orbital, then Nb is equal to the number of electrons in a bonding orbital.
Bond order =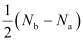
If Nb > Na, then the molecule is said be stable. However, if Nb ≤ Na, then the molecule is considered to be unstable.
Bond order of N2 can be calculated from its electronic configuration as:
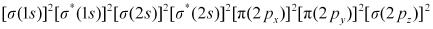
Number of bonding electrons, Nb = 10
Number of anti-bonding electrons, Na = 4
Bond order of nitrogen molecule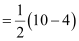
= 3
There are 16 electrons in a dioxygen molecule, 8 from each oxygen atom. The electronic configuration of oxygen molecule can be written as:
.gif)
Since the 1s orbital of each oxygen atom is not involved in boding, the number of bonding electrons = 8 = Nb and the number of anti-bonding electrons = 4 = Na.
Bond order.gif)
.gif)
= 2
Hence, the bond order of oxygen molecule is 2.
Similarly, the electronic configuration of.gif) can be written as:
can be written as:
.gif)
Nb = 8
Na = 3
Bond order of.gif)
= 2.5
Thus, the bond order of.gif) is 2.5.
is 2.5.
The electronic configuration of.gif) ion will be:
ion will be:
.gif)
Nb = 8
Na = 5
Bond order of.gif) =
= .gif)
= 1.5
Thus, the bond order of.gif) ion is 1.5.
ion is 1.5.