NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #38Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
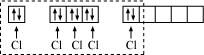
Ans : The ground state and excited state outer electronic configurations of phosphorus (Z = 15) are:
.png)
Phosphorus atom is sp3d hybridized in the excited state. These orbitals are filled by the electron pairs donated by five Cl atoms as:
PCl5
The five sp3d hybrid orbitals are directed towards the five corners of the trigonal bipyramidals. Hence, the geometry of PCl5 can be represented as:
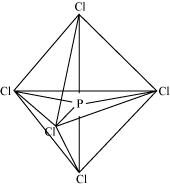
There are five P-Cl sigma bonds in PCl5. Three P-Cl bonds lie in one plane and make an angle of 120° with each other. These bonds are called equatorial bonds.
The remaining two P-Cl bonds lie above and below the equatorial plane and make an angle of 90° with the plane. These bonds are called axial bonds.
As the axial bond pairs suffer more repulsion from the equatorial bond pairs, axial bonds are slightly longer than equatorial bonds.