NEET-XI-Chemistry
04: Chemical Bonding and Molecular Structure
- #35Use molecular orbital theory to explain why the Be2 molecule does not exist.
Ans : The electronic configuration of Beryllium is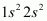 .
.
The molecular orbital electronic configuration for Be2 molecule can be written as:
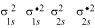
Hence, the bond order for Be2 is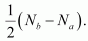
Where,
Nb = Number of electrons in bonding orbitals
Na = Number of electrons in anti-bonding orbitals
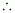 Bond order of Be2
Bond order of Be2 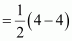 = 0
= 0
A negative or zero bond order means that the molecule is unstable. Hence, Be2 molecule does not exist.