NEET-XI-Chemistry
01: Some Basic Concepts of Chemistry
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- #Page No 22:
- Qstn #1-iH2O (ii) CO2 (iii) CH4Ans : H2O:
The molecular mass of water, H2O
= (2 × Atomic mass of hydrogen) + (1 × Atomic mass of oxygen)
= [2(1.0084) + 1(16.00 u)]
= 2.016 u + 16.00 u
= 18.016
= 18.02 u
(ii) CO2:
The molecular mass of carbon dioxide, CO2
= (1 × Atomic mass of carbon) + (2 × Atomic mass of oxygen)
= [1(12.011 u) + 2 (16.00 u)]
= 12.011 u + 32.00 u
= 44.01 u
(iii) CH4:
The molecular mass of methane, CH4
= (1 × Atomic mass of carbon) + (4 × Atomic mass of hydrogen)
= [1(12.011 u) + 4 (1.008 u)]
= 12.011 u + 4.032 u
= 16.043 u
- Qstn #2Calculate the mass percent of different elements present in sodium sulphate (Na2SO4).
Ans : The molecular formula of sodium sulphate is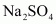 .
.
Molar mass of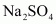 = [(2 × 23.0) + (32.066) + 4 (16.00)]
= [(2 × 23.0) + (32.066) + 4 (16.00)]
= 142.066 g
Mass percent of an element
∴ Mass percent of sodium:
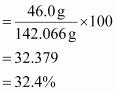
Mass percent of sulphur:
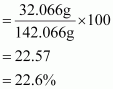
Mass percent of oxygen:
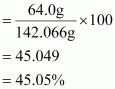
- Qstn #3Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.
Ans : % of iron by mass = 69.9 % [Given]
% of oxygen by mass = 30.1 % [Given]
Relative moles of iron in iron oxide:
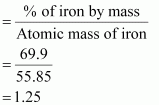
Relative moles of oxygen in iron oxide:
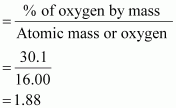
Simplest molar ratio of iron to oxygen:
= 1.25: 1.88
= 1: 1.5
 2: 3
2: 3
∴ The empirical formula of the iron oxide is Fe2O3.
- Qstn #4Calculate the amount of carbon dioxide that could be produced when
Ans : The balanced reaction of combustion of carbon can be written as:
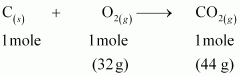
- Qstn #4-i1 mole of carbon is burnt in air.Ans : As per the balanced equation, 1 mole of carbon burns in1 mole of dioxygen (air) to produce1 mole of carbon dioxide.
- Qstn #4-ii1 mole of carbon is burnt in 16 g of dioxygen.Ans : According to the question, only 16 g of dioxygen is available. Hence, it will react with 0.5 mole of carbon to give 22 g of carbon dioxide. Hence, it is a limiting reactant.
- Qstn #4-iii2 moles of carbon are burnt in 16 g of dioxygen.Ans : According to the question, only 16 g of dioxygen is available. It is a limiting reactant. Thus, 16 g of dioxygen can combine with only 0.5 mole of carbon to give 22 g of carbon dioxide.
- Qstn #5Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0.375 molar aqueous solution. Molar mass of sodium acetate is 82.0245 g mol-1
Ans : 0.375 M aqueous solution of sodium acetate
≡ 1000 mL of solution containing 0.375 moles of sodium acetate
∴Number of moles of sodium acetate in 500 mL
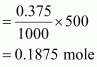
Molar mass of sodium acetate = 82.0245 g mole-1 (Given)
∴ Required mass of sodium acetate = (82.0245 g mol-1) (0.1875 mole)
= 15.38 g
- Qstn #6Calculate the concentration of nitric acid in moles per litre in a sample which has a density, 1.41 g mL-1 and the mass per cent of nitric acid in it being 69%.
Ans : Mass percent of nitric acid in the sample = 69 % [Given]
Thus, 100 g of nitric acid contains 69 g of nitric acid by mass.
Molar mass of nitric acid (HNO3)
= {1 + 14 + 3(16)} g mol-1
= 1 + 14 + 48
= 63 g mol-1
∴ Number of moles in 69 g of HNO3
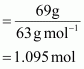
Volume of 100g of nitric acid solution
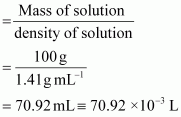
Concentration of nitric acid

∴Concentration of nitric acid = 15.44 mol/L
- Qstn #7How much copper can be obtained from 100 g of copper sulphate (CuSO4)?
Ans : 1 mole of CuSO4 contains 1 mole of copper.
Molar mass of CuSO4 = (63.5) + (32.00) + 4(16.00)
= 63.5 + 32.00 + 64.00
= 159.5 g
159.5 g of CuSO4 contains 63.5 g of copper.
⇒ 100 g of CuSO4 will contain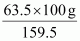 of copper.
of copper.
∴ Amount of copper that can be obtained from 100 g CuSO4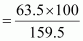
= 39.81 g
- Qstn #8Determine the molecular formula of an oxide of iron in which the mass per cent of iron and oxygen are 69.9 and 30.1 respectively. Given that the molar mass of the oxide is 159.69 g mol-1.
Ans : Mass percent of iron (Fe) = 69.9% (Given)
Mass percent of oxygen (O) = 30.1% (Given)
Number of moles of iron present in the oxide
= 1.25
Number of moles of oxygen present in the oxide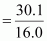
= 1.88
Ratio of iron to oxygen in the oxide,

= 1 : 1.5
= 2 : 3
; The empirical formula of the oxide is Fe2O3.
Empirical formula mass of Fe2O3 = [2(55.85) + 3(16.00)] g
Molar mass of Fe2O3 = 159.69 g
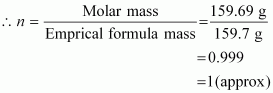
Molecular formula of a compound is obtained by multiplying the empirical formula with n.
Thus, the empirical formula of the given oxide is Fe2O3 and n is 1.
Hence, the molecular formula of the oxide is Fe2O3.
- Qstn #9Calculate the atomic mass (average) of chlorine using the following data:
% Natural Abundance Molar Mass 35Cl 75.77 34.9689 37Cl 24.23 36.9659 Ans : The average atomic mass of chlorine
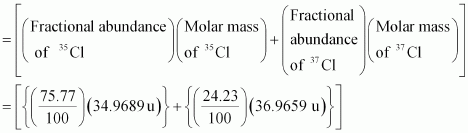
= 26.4959 + 8.9568
= 35.4527 u
∴ The average atomic mass of chlorine = 35.4527 u