NEET-XI-Chemistry
01: Some Basic Concepts of Chemistry
- #4Calculate the amount of carbon dioxide that could be produced when
() 1 mole of carbon is burnt in air.
() 1 mole of carbon is burnt in 16 g of dioxygen.
() 2 moles of carbon are burnt in 16 g of dioxygen.
() 1 mole of carbon is burnt in air.
() 1 mole of carbon is burnt in 16 g of dioxygen.
() 2 moles of carbon are burnt in 16 g of dioxygen.Ans : The balanced reaction of combustion of carbon can be written as:
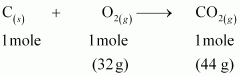
() As per the balanced equation, 1 mole of carbon burns in1 mole of dioxygen (air) to produce1 mole of carbon dioxide.
() According to the question, only 16 g of dioxygen is available. Hence, it will react with 0.5 mole of carbon to give 22 g of carbon dioxide. Hence, it is a limiting reactant.
() According to the question, only 16 g of dioxygen is available. It is a limiting reactant. Thus, 16 g of dioxygen can combine with only 0.5 mole of carbon to give 22 g of carbon dioxide.
() As per the balanced equation, 1 mole of carbon burns in1 mole of dioxygen (air) to produce1 mole of carbon dioxide.
() According to the question, only 16 g of dioxygen is available. Hence, it will react with 0.5 mole of carbon to give 22 g of carbon dioxide. Hence, it is a limiting reactant.
() According to the question, only 16 g of dioxygen is available. It is a limiting reactant. Thus, 16 g of dioxygen can combine with only 0.5 mole of carbon to give 22 g of carbon dioxide.
- #4-i1 mole of carbon is burnt in air.Ans : As per the balanced equation, 1 mole of carbon burns in1 mole of dioxygen (air) to produce1 mole of carbon dioxide.
- #4-ii1 mole of carbon is burnt in 16 g of dioxygen.Ans : According to the question, only 16 g of dioxygen is available. Hence, it will react with 0.5 mole of carbon to give 22 g of carbon dioxide. Hence, it is a limiting reactant.
- #4-iii2 moles of carbon are burnt in 16 g of dioxygen.Ans : According to the question, only 16 g of dioxygen is available. It is a limiting reactant. Thus, 16 g of dioxygen can combine with only 0.5 mole of carbon to give 22 g of carbon dioxide.