NEET-XI-Chemistry
01: Some Basic Concepts of Chemistry
- #7How much copper can be obtained from 100 g of copper sulphate (CuSO4)?
Ans : 1 mole of CuSO4 contains 1 mole of copper.
Molar mass of CuSO4 = (63.5) + (32.00) + 4(16.00)
= 63.5 + 32.00 + 64.00
= 159.5 g
159.5 g of CuSO4 contains 63.5 g of copper.
⇒ 100 g of CuSO4 will contain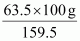 of copper.
of copper.
∴ Amount of copper that can be obtained from 100 g CuSO4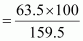
= 39.81 g