NEET-XI-Chemistry
01: Some Basic Concepts of Chemistry
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Qstn #10-iNumber of moles of carbon atoms.Ans : 1 mole of C2H6 contains 2 moles of carbon atoms.
∴ Number of moles of carbon atoms in 3 moles of C2H6
= 2 × 3 = 6
- Qstn #10-iiNumber of moles of hydrogen atoms.Ans : 1 mole of C2H6 contains 6 moles of hydrogen atoms.
∴ Number of moles of carbon atoms in 3 moles of C2H6
= 3 × 6 = 18
- Qstn #10-iiiNumber of molecules of ethane.Ans : 1 mole of C2H6 contains 6.023 × 1023 molecules of ethane.
∴ Number of molecules in 3 moles of C2H6
= 3 × 6.023 × 1023 = 18.069 × 1023
Page No 23:
- Qstn #11What is the concentration of sugar (C12H22O11) in mol L-1 if its 20 g are dissolved in enough water to make a final volume up to 2 L?
Ans : Molarity (M) of a solution is given by,
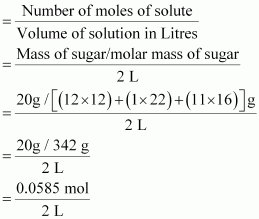
= 0.02925 mol L-1
∴ Molar concentration of sugar = 0.02925 mol L-1
- Qstn #12If the density of methanol is 0.793 kg L-1, what is its volume needed for making 2.5 L of its 0.25 M solution?
Ans : Molar mass of methanol (CH3OH) = (1 × 12) + (4 × 1) + (1 × 16)
= 32 g mol-1
= 0.032 kg mol-1
Molarity of methanol solution
= 24.78 mol L-1
(Since density is mass per unit volume)
Applying,
M1V1 = M2V2
(Given solution) (Solution to be prepared)
(24.78 mol L-1) V1 = (2.5 L) (0.25 mol L-1)
V1 = 0.0252 L
V1 = 25.22 mL
- Qstn #13Pressure is determined as force per unit area of the surface. The SI unit of pressure, Pascal is as shown below:
1Pa = 1N m-2
If mass of air at sea level is 1034 g cm-2, calculate the pressure in Pascal.
Ans : Pressure is defined as force acting per unit area of the surface.

= 1.01332 × 105 kg m-1s-2
We know,
1 N = 1 kg ms-2
Then,
1 Pa = 1 Nm-2 = 1 kg m-2s-2
1 Pa = 1 kg m-1s-2
∴ Pressure = 1.01332 × 105 Pa
- Qstn #14What is the SI unit of mass? How is it defined?
Ans : The SI unit of mass is kilogram (kg). 1 Kilogram is defined as the mass equal to the mass of the international prototype of kilogram.
- Qstn #15Match the following prefixes with their multiples:
Prefixes Multiples (i) micro 106 (ii) deca 109 (iii) mega 10-6 (iv) giga 10-15 (v) femto 10
Ans :Prefix Multiples (i) micro 10-6 (ii) deca 10 (iii) mega 106 (iv) giga 109 (v) femto 10-15
- Qstn #16What do you mean by significant figures?
Ans : Significant figures are those meaningful digits that are known with certainty.
They indicate uncertainty in an experiment or calculated value. For example, if 15.6 mL is the result of an experiment, then 15 is certain while 6 is uncertain, and the total number of significant figures are 3.
Hence, significant figures are defined as the total number of digits in a number including the last digit that represents the uncertainty of the result.
- Qstn #17A sample of drinking water was found to be severely contaminated with chloroform, CHCl3, supposed to be carcinogenic in nature. The level of contamination was 15 ppm (by mass).
- Qstn #17-iExpress this in percent by mass.Ans : 1 ppm is equivalent to 1 part out of 1 million (106) parts.
∴ Mass percent of 15 ppm chloroform in water
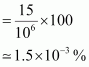
- Qstn #17-iiDetermine the molality of chloroform in the water sample.Ans : 100 g of the sample contains 1.5 × 10-3 g of CHCl3.
⇒ 1000 g of the sample contains 1.5 × 10-2 g of CHCl3.
∴ Molality of chloroform in water
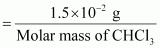
Molar mass of CHCl3 = 12.00 + 1.00 + 3(35.5)
= 119.5 g mol-1
∴ Molality of chloroform in water = 0.0125 × 10-2 m
= 1.25 × 10-4 m