NEET-XII-Chemistry
Previous Year Paper year:2017
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #46The number of sigma (``\sigma``) and pi (``\pi``) bonds in
pent-2-en-4-yne is
(1) 10 ``\sigma`` bonds and 3``\pi`` bonds
(2) 8 ``\sigma``bonds and 5``\pi`` bonds
(3) 11 ``\sigma`` bonds and 2``\pi`` bonds
(4) 13``\sigma`` bonds and no ``\pi`` bondsdigAnsr: 1Ans : ( 1 )
Sol. H - C - C = C - C C - H
H
H H
H
Number of bonds = 10
and number of bonds = 3
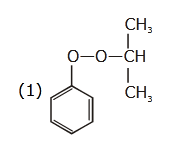
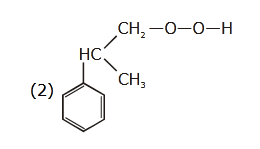
- Qstn #47The structure of intermediate A in the
following reaction, is
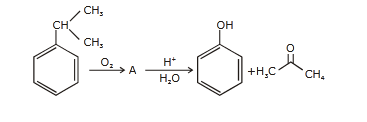
(1)
(2)
(3)
(4)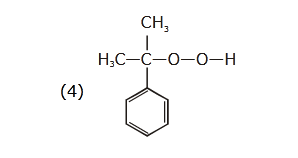 digAnsr: 4Ans : ( 4 )
digAnsr: 4Ans : ( 4 )
Sol.
CH
CH
3
CH
3
O2 H
+
H O2
OH
+ H C - C -CH3 3
O
H C - C - O - O - H3
CH
3
(A)
Cumene
hydroperoxide
Cumene
- Qstn #48The correct structure of tribromooctaoxide is
(1)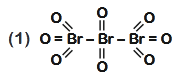
(2)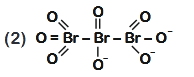
(3)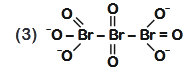
(4)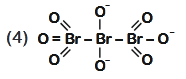 digAnsr: 1Ans : ( 1 )
digAnsr: 1Ans : ( 1 )
Sol. The correct structure is
O = Br - Br - Br = O
O
O
O
O
O
O
Tribromooctaoxide
- Qstn #494d, 5p, 5f and 6p orbitals are arranged in the
order of decreasing energy. The correct
option is
(1) 5f > 6p > 5p > 4d
(2) 6p > 5f > 5p > 4d
(3) 6p > 5f > 4d > 5p
(4) 5f > 6p > 4d > 5pdigAnsr: 1Ans : ( 1 )
Sol. (n + l) values for, 4d = 4 + 2 = 6
5p = 5 + 1 = 6
5f = 5 + 3 = 8
6p = 6 + 1 = 7
∴ Correct order of energy would be
5f > 6p > 5p > 4d
- Qstn #50Which of the following reactions are
disproportionation reaction?
(a )`` 2Cu^+ \longrightarrow`` ``Cu^{2+} + Cu^0``
(b)``3MnO_4^{2-} + 4H^+ \longrightarrow`` ``2MnO_4^- + MnO_2 + 2H_2O``
(c)\begin{aligned}
& \ce{$2KMnO_4 $ ->[\ce{\triangle}] $K_2MnO_4 + MnO_2 + 0_2$}
\end{aligned}
(d)``2MnO_4 ^- + 3Mn^{2+} + 2H_2O \longrightarrow`` ``5MnO_2 + 4H^+``
Select the correct option from the following
(1) (a) and (b) only
(2) (a), (b) and (c)
(3) (a), (c) and (d)
(4) (a) and (d) onlydigAnsr: 1Ans : ( 1 )
Sol. (a)
+
+
1
1
2Cu
+
+ +
2 0
2( ) 0
Cu Cu } Disproportionation
(b)
+
++
6
2( ) ( )
43MnO 4H
+ +
+ +
7 4
4 2 2
2MnO MnO 2H O}Disproportionation
(c)
+ +
▵ +
7 6
2
4 2 4
2KMnO K MnO
+
+ ∴
4 0
2 2
MnO O } Not a disproportionation
(d)
+ +
++ +
7 2
2( )
4 22MnO 3Mn 2H O
+
+
4
2
5MnO 4H }
14
- Qstn #51Under isothermal condition, a gas at 300 K
expands from 0.1 L to 0.25 L against a
constant external pressure of 2 bar. The work
done by the gas is
(Given that 1 L bar = 100 J)
(1) -30 J (2) 5 kJ
(3) 25 J (4) 30 JdigAnsr: 1Ans : ( 1 )
Sol. ∴ W
irr
= - P
ext
▵V
= - 2 bar × (0.25 - 0.1) L
= - 2 × 0.15 L-bar
= - 0.30 L-bar
= - 0.30 × 100 J
= - 30 J
- Qstn #52Among the following, the one that is not a
green house gas is
(1) Nitrous oxide (2) Methane
(3) Ozone (4) Sulphur dioxidedigAnsr: 4Ans : ( 4 )
Sol. Fact
SO
2
(g) is not a greenhouse gas.
- Qstn #53For the cell reaction
``2Fe^{3+}(aq) + 2I^- (aq) ````\longrightarrow`` ``2Fe^{2+}(aq) + I_2 (aq) ``
``E_{CELL}^\circleddash ``= 0.24 V at 298 K. The standard Gibbs
energy (``\triangle_rG^\circleddash ``)of the cell reaction is :
[Given that Faraday constant F = 96500 C `` mol^{-1}``]
(1) - 46.32 kJ `` mol^{-1}`` (2) - 23.16 kJ `` mol^{-1}``
(3) 46.32 kJ `` mol^{-1}`` (4) 23.16 kJ `` mol^{-1}``digAnsr: 1Ans : ( 1 )
Sol.
Θ Θ▵
cell
G = nF E
= - 2 × 96500 × 0.24 J mol-1
= - 46320 J mol-1
= - 46.32 kJ mol-1
- Qstn #54Enzymes that utilize ATP in phosphate transfer
require an alkaline earth metal (M) as the
cofactor. M is :
(1) Be (2) Mg
(3) Ca (4) SrdigAnsr: 2Ans : ( 2 )
Sol. All enzymes that utilize ATP in phosphate
transfer require magnesium(Mg) as the
co-factor.
- Qstn #55The most suitable reagent for the following
conversion, is :
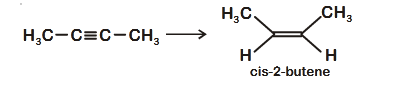
(1) Na/liquid ``NH_3``
(2) ``H_2``, Pd/C, quinoline
(3) Zn/HCl
(4) ``Hg^{2+}/H^+, H_2O``digAnsr: 2Ans : ( 2 )
Sol. H C C C CH3 3
CH
3
H
H C
3
H
cis-2-butene
H , Pd/C,2
quinoline
C C
- Qstn #56Which is the correct thermal stability order
for ``H_2E ``(E = O, S, Se, Te and Po)?
(1)`` H_2S < H_2O < H_2Se < H_2Te < H_2Po``
(2)`` H_2O < H_2S < H_2Se < H_2Te < H_2Po``
(3)`` H_2Po < H_2Te < H_2Se < H_2S < H_2O``
(4) ``H_2Se < H_2Te < H_2Po < H_2O < H_2S``digAnsr: 3Ans : ( 3 )
Sol. On going down the group thermal stability
order for H
2
E decreases because H-E bond
energy decreases
∴ Order of stability would be:-
H
2
Po < H
2
Te < H
2
Se < H
2
S < H
2
O
- Qstn #57Which of the following is incorrect statement?
(1)`` PbF_4`` is covalent in nature
(2) ``SiCl_4`` is easily hydrolysed
(3) ``GeX_4``(X = F, Cl, Br, I) is more stable than
``GeX_2``
(4)`` SnF_4`` is ionic in nature.digAnsr: 1Ans : ( 1 )
Sol. PbF
4
and SnF
4
are ionic in nature.
- Qstn #58Match the following :
(a ) Pure nitrogen (i) Chlorine
(b) Haber process (ii) Sulphuric acid
(c) Contact process (iii) Ammonia
(d) Deacon’s process (iv) Sodium azide or Barium azide
Which of the following is the correct option?
(a ) (b) (c) (d)
(1) (i) (ii) (iii) (iv)
(2) (ii) (iv) (i) (iii)
(3) (iii) (iv) (ii) (i)
(4) (iv) (iii) (ii) (i)digAnsr: 4Ans : ( 4 )
Sol. (a) Pure nitrogen : Sodium azide or
Barium azide
(b) Haber process : Ammonia
(c) Contact process : Sulphuric acid
(d) Deacon’s process : Chlorine
- Qstn #59Which of the following diatomic molecular
species has only ``\PI`` bonds according to
Molecular Orbital Theory?
(1) ``O_2``
(2)`` N_2``
(3)`` C_2``
(4) ``Be_2``digAnsr: 3Ans : ( 3 )
Sol. MO configuration C
2
is:
1s2, *1s2, 2s2, *2s2, = 2 2x y2p 2p
- Qstn #60For the second period elements the correct
increasing order of first ionisation enthalpy is:
(1) Li < Be < B < C < N < O < F < Ne
(2) Li < B < Be < C < O < N < F < Ne
(3) Li < B < Be < C < N < O < F < Ne
(4) Li < Be < B < C < O < N < F < NedigAnsr: 2Ans : ( 2 )
Sol. ‘Be’ and ‘N’ have comparatively more stable
valence sub-shell than ‘B’ and ‘O’.
∴ Correct order of first ionisation enthalpy
is:
Li < B < Be < C < O < N < F < Ne