NEET-XII-Chemistry
Previous Year Paper year:2017
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #1The most suitable method of separation of
1 : 1 mixture of ortho and para-nitrophenols is :
(1) Steam distillation
(2) Sublimation
(3) Chromatography
(4) CrystallisationdigAnsr: 1Ans : (1)
Sol. In O-nitrophenol intra molecular H-Bond present. So Bpt is low where as in p-nitrophenol molecules are
associated by inter molecular H-Bond. So Bpt is high, so o & p-nitrophenol seperated by steam distillation
method
O
H
δ+
N = O
↓ δ-
O
O - nitrophenol
N
O
O ---- H
O
H
NO
O
O ---- H
N O
O
O ---- H
P-nitrophenol
- Qstn #2Which of the following statements is
not correct?
(1) Denaturation makes the proteins more active.
(2) Insulin maintains sugar level in the blood of a human body.
(3) Ovalbumin is a simple food reserve in egg-white.
(4) Blood proteins thrombin and fibrinogen are involved in blood clotting.digAnsr: 1Ans : (1)
Sol. ∵ Denaturation makes the protein inactive.
CAREER POINT







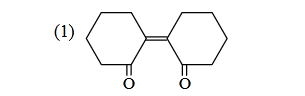
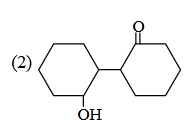
- Qstn #3Of the following, which is the product
formed when cyclohexanone undergoes aldol
condensation followed by heating?
(1)
(2)
(3)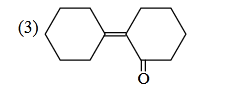
(4)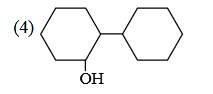 digAnsr: 3Ans : (3)
digAnsr: 3Ans : (3)
Sol.
O
O + H
H
α
)OH(-
onCondensati
aldol
/OH
2
⎯⎯ →⎯ Δ
Θ
O
- Qstn #4The heating of phenyl-methyl ethers
with HI produces.
(1) benzene
(2) ethyl chlorides
(3) iodobenzene
(4) phenoldigAnsr: 4Ans : (4)
Sol.
O - CH3 2SN
HI⎯→⎯
Methyl Phenyl
ether
Phenol
OH + CH3I
Methyl iodide
- Qstn #5The correct increasing order of basic
strength for the following compounds is :
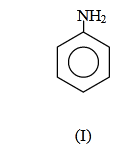
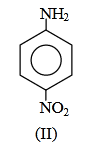
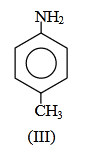
(1) II < I < III
(2) II < III < I
(3) III < I < II
(4) III < II < IdigAnsr: 1Ans : (1)









Sol. ∵ Basic strength ∝
I-,H-,M-
I,H,M +++
∴
Answer will be : NH2 NH2
NO2
NH2
CH3
(I) (II)(III)
〉〉
- Qstn #6Which one of the following pairs of
species have the same bond order?
(1) ``N_2``, ``O_2^{-}``
(2) CO, NO
(3)`` O_2``, NO
(4) ``CN^-, COdigAnsr: 4Ans : (4)
Sol. CO & CN- are isoelectronic and having same bond order 3
- Qstn #7Name the gas that can readily
decolourise acidified KMnO4 solution :
(1) ``P_2O_5 ``
(2) ``CO_2``
(3) ``SO_2``
(4) ``NO_2``digAnsr: 3Ans : (3)
Sol. KMnO4 + SO2 + H2SO4 → K2SO4 + MnSO4 + H2O
∴ SO2 which is R.A. decolourize KMnO4
- Qstn #8The reason for greater range of oxidation
states in actinoids is attributed to :
(1) 4f and 5d levels being close in energies
(2) the radioactive nature of actinoids
(3) actinoid contraction
(4) 5f, 6d and 7s levels having comparable energiesdigAnsr: 4Ans : (4)
Sol. Actinoid shows grater range of oxidation state because 5f, 6d, 7s levels having comparable energies.
- Qstn #9Concentration of the ``Ag^+`` ions in a saturated
solution of ``Ag_2C_2O_4`` is 2.2 × ``10^{-4}`` mol ``L^{-1}``.
Solubility product of ``Ag_2C_2O_4`` is :
(1) 5.3 × ``10^{-12}``
(2) 2.42 × ``10^{-8}``
(3) 2.66 × ``10^{-12}``
(4) 4.5 × ``10^{-11}``digAnsr: 1Ans : (1)




Sol. Ag2C2O4
s2
Ag2 + +
s
OC 2-42
2s = 2.2 × 10-4
s = 1.1 × 10-4
Ksp = 4s3 = 4 × (1.1 × 10-4)3
= 4 × (1.1)3 × 10-12
= 5.324 × 10-12 M3
- Qstn #10With respect to the conformers of ethane,
which of the following statements is true?
(1) Both bond angles and bond length remains same
(2) Bond angle remains same but bond length changes
(3) Bond angle changes but bond length remains same
(4) Both bond angle and bond length changedigAnsr: 1Ans : (1)
Sol.
H
H H
H H
H
Bond angle ⇒ Unchanged
Bond length ⇒ Unchanged
H HH
H
H
H
- Qstn #11Identify A and predict the type of reaction-
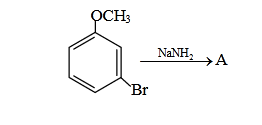
(1)
(2)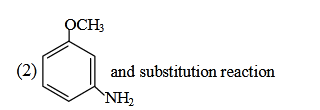
(3)
(4)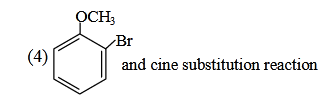 digAnsr: 2Ans : (2)
digAnsr: 2Ans : (2)








Sol.
OCH3
Br
)NH)(-NaBr(-
NaNH
3
2⎯⎯ →⎯
H
OCH3
⎯⎯ →⎯ 3HN
OCH3
NH3
Θ
⊕
OCH3
NH2
..
Intra mol.
Proton transfer
Overall Br is replaced by NH2 group so we can say substitution reaction.
- Qstn #12Which of the following is sink for CO?
(1) Plants
(2) Haemoglobin
(3) Micro organisms present in the soil
(4) OceansdigAnsr: 3Ans : (3)
Sol. Soil is a natural sink for carbon monoxide. The soil's ability to remove carbon monoxide from the
atmosphere is due to the activity of soil micro-organisms.
- Qstn #13In which pair of ions both the species
contain S - S bond?
(1) ``S_4O_6^{2-}`` , ``S_2O_7^{2-}``
(2)``S_2O_7^{2-}`` , ``S_2O_3^{2-}``
(3)``S_4O_6^{2-}`` , ``S_2O_3^{2-}``
(4)``S_2O_7^{2-}`` , ``S_2O_8^{2-}``digAnsr: 3Ans : (3)
Sol.
(i)
O - S - S - S - S - O
O
O
O
O
S4O6-2
O - S - O
O
S
S2O3-2
- Qstn #14Pick out the correct statement with respect to ``[Mn(CN)_6]^{3-}`` :
(1) It is ``dsp^2`` hybridised and square planar
(2) It is ``sp^3d^2`` hybridised and octahedral
(3) It is ``sp^3d^2`` hybridised and tetrahedral
(4) It is ``d^2sp^3`` hybridised and octahedraldigAnsr: 4Ans : (4)
Sol. [Mn(CN)6]-3


25Mn = [Ar] 3d54s2
Mn+3 = [Ar] 3d4
CN- is strong ligand
∴ Δ0 is high complex is d2sp3 hybridised and inner octahedral
d2 sp3
inner octahedral
n = 2
- Qstn #15The equilibrium constants of the following are :
``\ce [N2 +3H2 \rightleftarrows 2NH3``]
``\ce [N2 + O2 \rightleftarrows 2NO``]
``\ce [H2 + \frac12 O2 \rightleftarrows H2O``]
The equilibrium constant (K) of the reaction :
``\ce 2NH_3 + \frac52 O_2`` ``\xrightleftharpoons{K}`` ``2NO + 3H_2O``
, will be:
(1) ``K_2^3`` ``K_3/ K_1``
(2)``K_1`` ``K_3^3``/``K_2``
(3) ``K_2`` ``K_3^3``/``K_1``
(4) ``K_2`` ``K_3``/``K_1``digAnsr: 3Ans : (3)
Sol. (1) N2 + 3 H2 2 NH3 K1
(2) N2 + O2 2 NO K2
(3) H2 + 2
1 O2 H2O K3
Object 2 NH3 + 2
5 O2
K 2 NO + 3 H2O
Equation (3) × 3 + (2) - (1)
∴ K =
1
3
32
K
KK ×