NEET-XII-Chemistry
Previous Year Paper year:2018
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
- Qstn #60Which one is a wrong statement ?
(1) Total orbital angular momentum of electron in
's' orbital is equal to zero
(2) An orbital is designated by three quantum
numbers while an electron in an atom is
designated by four quantum numbers.
(3) The electronic configuration of N atom is
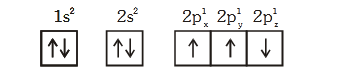
(4) The value of m for ``dz^2`` is zerodigAnsr: 3Ans : (3)
Sol. The correct configuration of 'N' is
- Qstn #61Consider the following species:
``CN^+, CN^-, NO and CN``
Which one of these will have the highest bond order?
(1)`` NO ``
(2)`` CN^-``
(3) ``CN^+ ``
(4) CNdigAnsr: 2Ans : (2)
Sol. Ion/Species Total electron Bond order
NO 15 2.5
CN- 14 3
CN+ 12 2
CN 13 2.5
- Qstn #62Which of the following statements is not true for
halogens ?
(1) All form monobasic oxyacids.
(2) All are oxidizing agents.
(3) All but fluorine show positive oxidation states.
(4) Chlorine has the highest electron-gain enthalpy.digAnsr: BAns : (Bonus)
- Qstn #63Which one of the following elements is unable to
form ``MF_6^{3-}``?
(1) Ga (2) AI (3) B (4) IndigAnsr: 3Ans : (3)
Sol. MF
6
-3
Boron belongs to 2nd period and it does not have
vacant d-orbital.
- Qstn #64In the structure of ``ClF_3``, the number of lone pairs
of electrons on central atom 'Cl' is
(1) one
(2) two
(3) four
(4) threedigAnsr: 2Ans : (2)
Sol. Cl - F
F
F
2 lone pair at equitorial position.
- Qstn #65Considering Ellingham diagram, which of the
following metals can be used to reduce alumina ?
(1) Fe
(2) Zn
(3) Mg
(4) CudigAnsr: 3Ans : (3)
Sol. Mg has more -▵G value then alumina. So it will be
in the lower part of Ellingham diagram. Metals
which has more -▵G value can reduce those metals
oxide which has less -▵G value.
- Qstn #66The correct order of atomic radii in group 13
elements is
(1) B < Al < In < Ga < Tl
(2) B < Al < Ga < In < Tl
(3) B < Ga < Al < Tl < In
(4) B < Ga < Al < In < TldigAnsr: 4Ans : (4)
Sol. In group 13 due to transition contraction [Al > Ga]
- Qstn #67The correct order of N-compounds in its decreasing
order of oxidation states is
``\ce (1) HNO3, NO, N2, NH4Cl``
``\ce (2) HNO3, NO, NH4Cl, N2``
``\ce (3) HNO3, NH4Cl, NO, N2``
``\ce (4) NH4Cl, N2, NO, HNO3``digAnsr: 1Ans : (1)
Sol.
5
3HNO
+
,
2
NO
+
,
0
2N ,
3
4NH Cl
- Qstn #68On which of the following properties does
coagulating power of an ion depend ?
(1) The magnitude of the charge on the alone
(2) Size of the ion alone
(3) Both magnitude and sign of the charge the ion
(4) The sign of charge on the ion alonedigAnsr: 3Ans : (3)
Sol. According to Hardy Schulze rule : The coagulating
power of an ion depend on both magnitude and sign
of the charge of the ion.
- Qstn #69Following solutions were prepared by mixing
different volumes of NaOH and HCl of different
concentrations :
a. 60mL ``\frac{M}{10}``HCl +
40mL ``\frac{M}{10}`` NaOH
b. 55mL ``\frac{M}{10}``HCl +
45mL ``\frac{M}{10}`` NaOH
c. 75mL ``\frac{M}{5}``HCl +
25mL ``\frac{M}{5}`` NaOH
d. 100mL ``\frac{M}{10}``HCl +
100mL ``\frac{M}{10}`` NaOH
pH of which one of them will be equal to 1 ?
(1) b
(2) a
(3) d
(4) cdigAnsr: 4Ans : (4)
Sol. As N1V1 > N2V2
So acid is left at the end of reaction
Nfinal solution = [H
+] =
1 1 2 2
1 2
N V N V
V V
+
=
1 1
75 25
5 5
75 25
+
=
1
0.1
10
=
pH = -log[H+] = 1
14
NEET(UG)-2018
- Qstn #70The solubility of ``BaSO_4`` in water
2.42 × 103 ``gL^{-1}``at 298 K. The value
of solubility product (``K_{sp}``) will be
(Given molar mass of BaSO4 = 233 g ``mol ^{-1}``
(1) ``1.08 × 10^{-10} mol^2 L^{-2}``
(2)`` 1.08 × 10^{-12} mol^2 L^{-2}``
(3)`` 1.08 × 10^{-14} mol^2 L^{-2}``
(4)`` 1.08 × 10^{-8} mol^2 L^{-2}``digAnsr: 1Ans : (1)
Sol. solubility of BaSO4 = 2.42 × 10-3 gL-1
∴ s =
=
3
5 12.42 10 1.038 10 mol L
233
Ksp = s
2 = (1.038 × 10-5)2
= 1.08 × 10-10 mol2 L-2
- Qstn #71Given van der Waals constant for ``\ce NH3, H2`` and ``CO_2``
are respectively 4.17, 0.244, 1.36 and 3.59, which
one of the following gases is most easily liquefied?
(1) ``\ce NH3``
(2) ``H_2 ``
(3)`` O_2 ``
(4)`` CO_2``digAnsr: 1Ans : (1)
Sol. Critical temperature vanderwaal constant(a)
maximum "a" &implies; gas with maximum TC &implies; easiest
liquification = NH3
- Qstn #72The compound A on treatment with Na gives B, and
with ``PCl_5 ``gives C. B and C react together to give
diethyl ether. A, B and C are in the order
(1) ``\ce C2H5OH, C2H6, C2H5Cl``
(2) ``\ce C2H5OH, C2H5Cl, C2H5ONa``
(3) ``\ce C2H5Cl, C2H6, C2H5OH``
(4) ``\ce C2H5OH, C2H5ONa, C2H5Cl``digAnsr: 4Ans : (4)
Sol. C H OH 2 5 C H ONa2 5
Na
A B
C H OH 2 5 C H Cl2 5
PCl5
A C
C H ONa + 2 5 C H -Cl2 5
A C
SN
2
Williamson's
synthesis of ether
C H -O-C H2 5 2 5
diethylether
- Qstn #73Hydrocarbon (A) reacts with bromine by substitution
to form an alkyl bromide which by Wurtz reaction
is converted to gaseous hydrocarbon containing less
than four carbon atoms. (A) is
(1) CH ``\equiv `` CH
(2)`` CH_2=CH_2``
(3)`` CH_3-CH_3 ``
(4) ``CH_4``digAnsr: 4Ans : (4)
Sol.
CH4 CH -Br3
Br2 CH -CH 3 3
Na
ether
(less than four 'C')
h
- Qstn #74The compound ``\ce C7H8 ``undergoes the following
reactions :
``\ce{$C7H8$ ->[\ce{ \frac {3Cl_2}{\lambda}}] $A$} ``
``\ce{$$ ->[\ce{\frac {Br_2}{Fe}}] $B$} \\``
``\ce{$$ ->[\ce{\frac {Zn}{HCl}}] $C$} \\``
The product 'C' is
(1) m-bromotoluene
(2) o-bromotoluene
(3) 3-bromo-2,4,6-trichlorotoluene
(4) p-bromotoluenedigAnsr: 1Ans : (1)
Sol.
CH3
3Cl2
▵
CCl3
Br2
Fe
CCl3
Zn
HCl
Br
CH3
Br
m-bromotoluene