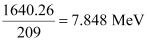NEET-XII-Physics
13: Nuclei
- #4Obtain the binding energy of the nuclei
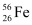 and
and 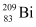 in units of MeV from the following data:
in units of MeV from the following data:
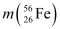 = 55.934939 u
= 55.934939 u 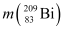 = 208.980388 u
= 208.980388 u
Ans : Atomic mass of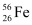 , m1 = 55.934939 u
, m1 = 55.934939 u
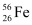 nucleus has 26 protons and (56 - 26) = 30 neutrons
nucleus has 26 protons and (56 - 26) = 30 neutrons
Hence, the mass defect of the nucleus, Δm = 26 × mH + 30 × mn - m1
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn = 1.008665 u
∴Δm = 26 × 1.007825 + 30 × 1.008665 - 55.934939
= 26.20345 + 30.25995 - 55.934939
= 0.528461 u
But 1 u = 931.5 MeV/c2
∴Δm = 0.528461 × 931.5 MeV/c2
The binding energy of this nucleus is given as:
Eb1 = Δmc2
Where,
c = Speed of light
∴Eb1 = 0.528461 × 931.5.gif)
= 492.26 MeV
Average binding energy per nucleon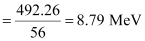
Atomic mass of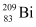 , m2 = 208.980388 u
, m2 = 208.980388 u
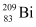 nucleus has 83 protons and (209 - 83) 126 neutrons.
nucleus has 83 protons and (209 - 83) 126 neutrons.
Hence, the mass defect of this nucleus is given as:
Δm‘ = 83 × mH + 126 × mn - m2
Where,
Mass of a proton, mH = 1.007825 u
Mass of a neutron, mn = 1.008665 u
∴Δm‘ = 83 × 1.007825 + 126 × 1.008665 - 208.980388
= 83.649475 + 127.091790 - 208.980388
= 1.760877 u
But 1 u = 931.5 MeV/c2
∴Δm‘ = 1.760877 × 931.5 MeV/c2
Hence, the binding energy of this nucleus is given as:
Eb2 = Δm‘c2
= 1.760877 × 931.5.gif)
= 1640.26 MeV
Average bindingenergy per nucleon =