NEET-XII-Physics
13: Nuclei
- #30Calculate and compare the energy released by a) fusion of 1.0 kg of hydrogen deep within Sun and b) the fission of 1.0 kg of 235U in a fission reactor.
Ans : a) Amount of hydrogen, m = 1 kg = 1000 g
1 mole, i.e., 1 g of hydrogen (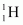 ) contains 6.023 × 1023 atoms.
) contains 6.023 × 1023 atoms.
∴1000 g of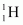 contains 6.023 × 1023 × 1000 atoms.
contains 6.023 × 1023 × 1000 atoms.
Within the sun, four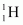 nuclei combine and form one
nuclei combine and form one .gif) nucleus. In this process 26 MeV of energy is released.
nucleus. In this process 26 MeV of energy is released.
Hence, the energy released from the fusion of 1 kg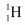 is:
is:
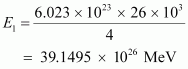
b) Amount of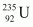 = 1 kg = 1000 g
= 1 kg = 1000 g
1 mole, i.e., 235 g of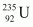 contains 6.023 × 1023 atoms.
contains 6.023 × 1023 atoms.
∴1000 g of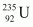 contains
contains 
It is known that the amount of energy released in the fission of one atom of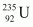 is 200 MeV.
is 200 MeV.
Hence, energy released from the fission of 1 kg of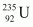 is:
is:
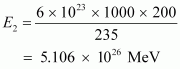
∴
Therefore, the energy released in the fusion of 1 kg of hydrogen is nearly 8 times the energy released in the fission of 1 kg of uranium.