NEET-XII-Physics
13: Nuclei
- #28Consider the D-T reaction (deuterium-tritium fusion)

(a) Calculate the energy released in MeV in this reaction from the data:
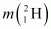 = 2.014102 u
= 2.014102 u
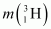 = 3.016049 u
= 3.016049 u
(b) Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction? (Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzman’s constant, T = absolute temperature.)
(a) Calculate the energy released in MeV in this reaction from the data:
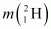 = 2.014102 u
= 2.014102 u
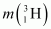 = 3.016049 u
= 3.016049 u
(b) Consider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction? (Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzman’s constant, T = absolute temperature.)Ans : null (a) Take the D-T nuclear reaction:
It is given that:
Mass of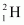 , m1= 2.014102 u
, m1= 2.014102 u
Mass of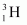 , m2 = 3.016049 u
, m2 = 3.016049 u
Mass of.gif) m3 = 4.002603 u
m3 = 4.002603 u
Mass of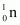 , m4 = 1.008665 u
, m4 = 1.008665 u
Q-value of the given D-T reaction is:
Q = [m1 + m2- m3 - m4] c2
= [2.014102 + 3.016049 - 4.002603 - 1.008665] c2
= [0.018883 c2] u
But 1 u = 931.5 MeV/c2
∴Q = 0.018883 × 931.5 = 17.59 MeV
(b) Radius of deuterium and tritium, r ≈ 2.0 fm = 2 × 10-15 m
Distance between the two nuclei at the moment when they touch each other, d = r + r = 4 × 10-15 m
Charge on the deuterium nucleus = e
Charge on the tritium nucleus = e
Hence, the repulsive potential energy between the two nuclei is given as:
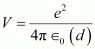
Where,
∈0 = Permittivity of free space
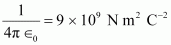

Hence, 5.76 × 10-14 J or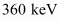 of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
However, it is given that:
KE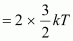
Where,
k = Boltzmann constant = 1.38 × 10-23 m2 kg s-2 K-1
T = Temperature required for triggering the reaction
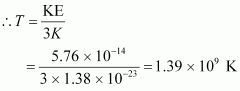
Hence, the gas must be heated to a temperature of 1.39 × 109 K to initiate the reaction.
(a) Take the D-T nuclear reaction:
It is given that:
Mass of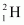 , m1= 2.014102 u
, m1= 2.014102 u
Mass of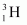 , m2 = 3.016049 u
, m2 = 3.016049 u
Mass of.gif) m3 = 4.002603 u
m3 = 4.002603 u
Mass of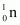 , m4 = 1.008665 u
, m4 = 1.008665 u
Q-value of the given D-T reaction is:
Q = [m1 + m2- m3 - m4] c2
= [2.014102 + 3.016049 - 4.002603 - 1.008665] c2
= [0.018883 c2] u
But 1 u = 931.5 MeV/c2
∴Q = 0.018883 × 931.5 = 17.59 MeV
(b) Radius of deuterium and tritium, r ≈ 2.0 fm = 2 × 10-15 m
Distance between the two nuclei at the moment when they touch each other, d = r + r = 4 × 10-15 m
Charge on the deuterium nucleus = e
Charge on the tritium nucleus = e
Hence, the repulsive potential energy between the two nuclei is given as:
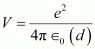
Where,
∈0 = Permittivity of free space
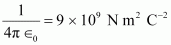

Hence, 5.76 × 10-14 J or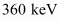 of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
However, it is given that:
KE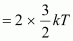
Where,
k = Boltzmann constant = 1.38 × 10-23 m2 kg s-2 K-1
T = Temperature required for triggering the reaction
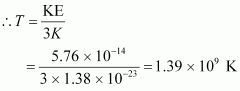
Hence, the gas must be heated to a temperature of 1.39 × 109 K to initiate the reaction.
- #28-aCalculate the energy released in MeV in this reaction from the data:
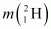 = 2.014102 u
= 2.014102 u
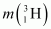 = 3.016049 u
= 3.016049 u
Ans : Take the D-T nuclear reaction:
It is given that:
Mass of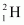 , m1= 2.014102 u
, m1= 2.014102 u
Mass of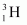 , m2 = 3.016049 u
, m2 = 3.016049 u
Mass of.gif) m3 = 4.002603 u
m3 = 4.002603 u
Mass of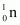 , m4 = 1.008665 u
, m4 = 1.008665 u
Q-value of the given D-T reaction is:
Q = [m1 + m2- m3 - m4] c2
= [2.014102 + 3.016049 - 4.002603 - 1.008665] c2
= [0.018883 c2] u
But 1 u = 931.5 MeV/c2
∴Q = 0.018883 × 931.5 = 17.59 MeV
- #28-bConsider the radius of both deuterium and tritium to be approximately 2.0 fm. What is the kinetic energy needed to overcome the coulomb repulsion between the two nuclei? To what temperature must the gas be heated to initiate the reaction? (Hint: Kinetic energy required for one fusion event =average thermal kinetic energy available with the interacting particles = 2(3kT/2); k = Boltzman’s constant, T = absolute temperature.)Ans : Radius of deuterium and tritium, r ≈ 2.0 fm = 2 × 10-15 m
Distance between the two nuclei at the moment when they touch each other, d = r + r = 4 × 10-15 m
Charge on the deuterium nucleus = e
Charge on the tritium nucleus = e
Hence, the repulsive potential energy between the two nuclei is given as:
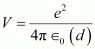
Where,
∈0 = Permittivity of free space
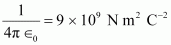

Hence, 5.76 × 10-14 J or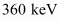 of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
of kinetic energy (KE) is needed to overcome the Coulomb repulsion between the two nuclei.
However, it is given that:
KE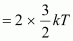
Where,
k = Boltzmann constant = 1.38 × 10-23 m2 kg s-2 K-1
T = Temperature required for triggering the reaction
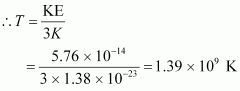
Hence, the gas must be heated to a temperature of 1.39 × 109 K to initiate the reaction.