NEET-XII-Physics
11: Dual Nature Of Radiation And Matter
- #4Monochromatic light of wavelength 632.8 nm is produced by a helium-neon laser. The power emitted is 9.42 mW.
(a) Find the energy and momentum of each photon in the light beam,
(b) How many photons per second, on the average, arrive at a target irradiated by this beam? (Assume the beam to have uniform cross-section which is less than the target area), and
(c) How fast does a hydrogen atom have to travel in order to have the same momentum as that of the photon?Ans : Wavelength of the monochromatic light, λ = 632.8 nm = 632.8 × 10-9 m
Power emitted by the laser, P = 9.42 mW = 9.42 × 10-3 W
Planck’s constant, h = 6.626 × 10-34 Js
Speed of light, c = 3 × 108 m/s
Mass of a hydrogen atom, m = 1.66 × 10-27 kg
(a) The energy of each photon is given as:
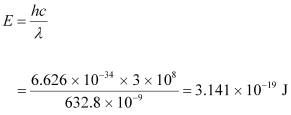
The momentum of each photon is given as:
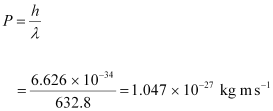
(b) Number of photons arriving per second, at a target irradiated by the beam = n
Assume that the beam has a uniform cross-section that is less than the target area.
Hence, the equation for power can be written as:
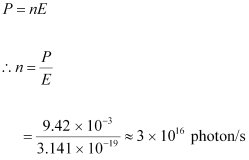
(c) Momentum of the hydrogen atom is the same as the momentum of the photon,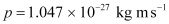
Momentum is given as:
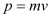
Where,
v = Speed of the hydrogen atom

- #4-aFind the energy and momentum of each photon in the light beam,Ans : The energy of each photon is given as:
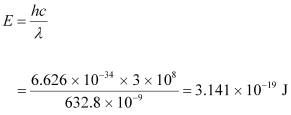
The momentum of each photon is given as:
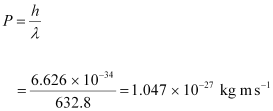
- #4-bHow many photons per second, on the average, arrive at a target irradiated by this beam? (Assume the beam to have uniform cross-section which is less than the target area), andAns : Number of photons arriving per second, at a target irradiated by the beam = n
Assume that the beam has a uniform cross-section that is less than the target area.
Hence, the equation for power can be written as:
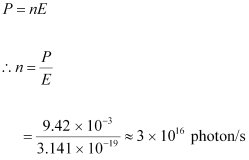
- #4-cHow fast does a hydrogen atom have to travel in order to have the same momentum as that of the photon?Ans : Momentum of the hydrogen atom is the same as the momentum of the photon,
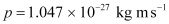
Momentum is given as:
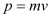
Where,
v = Speed of the hydrogen atom
