NEET-XII-Physics
11: Dual Nature Of Radiation And Matter
- #16An electron and a photon each have a wavelength of 1.00 nm. Find
(a) their momenta,
(b) the energy of the photon, and
(c) the kinetic energy of electron.Ans : Wavelength of an electron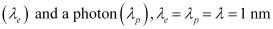
= 1 × 10-9 m
Planck’s constant, h = 6.63 × 10-34 Js
(a) The momentum of an elementary particle is given by de Broglie relation:
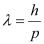
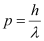
It is clear that momentum depends only on the wavelength of the particle. Since the wavelengths of an electron and a photon are equal, both have an equal momentum.
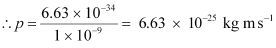
(b) The energy of a photon is given by the relation:
.gif)
Where,
Speed of light, c = 3 × 108 m/s
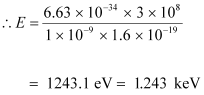
Therefore, the energy of the photon is 1.243 keV.
(c) The kinetic energy (K) of an electron having momentum p,is given by the relation:
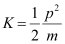
Where,
m = Mass of the electron = 9.1 × 10-31 kg
p = 6.63 × 10-25 kg m s-1
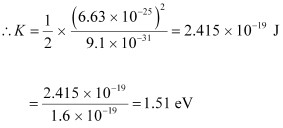
Hence, the kinetic energy of the electron is 1.51 eV.
- #16-atheir momenta,Ans : The momentum of an elementary particle is given by de Broglie relation:
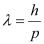
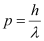
It is clear that momentum depends only on the wavelength of the particle. Since the wavelengths of an electron and a photon are equal, both have an equal momentum.
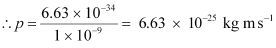
- #16-bthe energy of the photon, andAns : The energy of a photon is given by the relation:
.gif)
Where,
Speed of light, c = 3 × 108 m/s
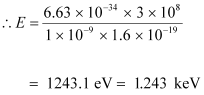
Therefore, the energy of the photon is 1.243 keV.
- #16-cthe kinetic energy of electron.Ans : The kinetic energy (K) of an electron having momentum p,is given by the relation:
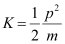
Where,
m = Mass of the electron = 9.1 × 10-31 kg
p = 6.63 × 10-25 kg m s-1
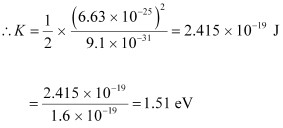
Hence, the kinetic energy of the electron is 1.51 eV.