NEET-XII-Physics
11: Dual Nature Of Radiation And Matter
- #17null (a) For what kinetic energy of a neutron will the associated de Broglie wavelength be 1.40 × 10-10 m?
(b) Also find the de Broglie wavelength of a neutron, in thermal equilibrium with matter, having an average kinetic energy of (3/2) kT at 300 K.Ans : null (a) De Broglie wavelength of the neutron, λ = 1.40 × 10-10 m
Mass of a neutron, mn = 1.66 × 10-27 kg
Planck’s constant, h = 6.6 × 10-34 Js
Kinetic energy (K) and velocity (v) are related as:
 ... (1)
... (1)
De Broglie wavelength (λ) and velocity (v) are related as:

Using equation (2) in equation (1), we get:
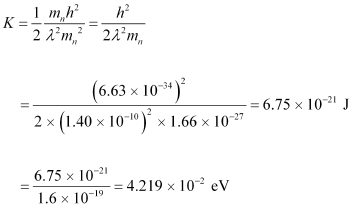
Hence, the kinetic energy of the neutron is 6.75 × 10-21 J or 4.219 × 10-2 eV.
(b) Temperature of the neutron, T = 300 K
Boltzmann constant, k = 1.38 × 10-23 kg m2 s-2 K-1
Average kinetic energy of the neutron:
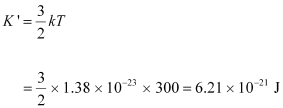
The relation for the de Broglie wavelength is given as:
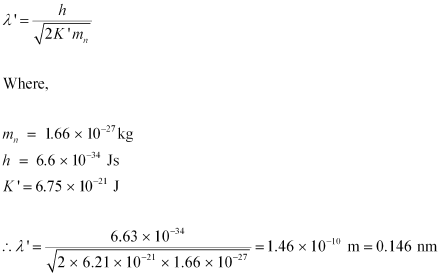
Therefore, the de Broglie wavelength of the neutron is 0.146 nm.
- #17-aFor what kinetic energy of a neutron will the associated de Broglie wavelength be 1.40 × 10-10 m?Ans : De Broglie wavelength of the neutron, λ = 1.40 × 10-10 m
Mass of a neutron, mn = 1.66 × 10-27 kg
Planck’s constant, h = 6.6 × 10-34 Js
Kinetic energy (K) and velocity (v) are related as:
 ... (1)
... (1)
De Broglie wavelength (λ) and velocity (v) are related as:

Using equation (2) in equation (1), we get:
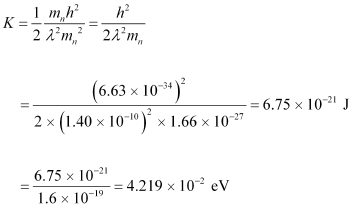
Hence, the kinetic energy of the neutron is 6.75 × 10-21 J or 4.219 × 10-2 eV.
- #17-bAlso find the de Broglie wavelength of a neutron, in thermal equilibrium with matter, having an average kinetic energy of (3/2) kT at 300 K.Ans : Temperature of the neutron, T = 300 K
Boltzmann constant, k = 1.38 × 10-23 kg m2 s-2 K-1
Average kinetic energy of the neutron:
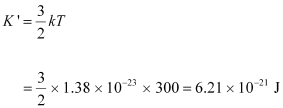
The relation for the de Broglie wavelength is given as:
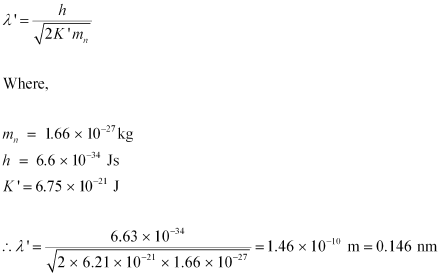
Therefore, the de Broglie wavelength of the neutron is 0.146 nm.