NEET-XII-Physics
11: Dual Nature Of Radiation And Matter
- #13What is the
(a) momentum,
(b) speed, and
(c) de Broglie wavelength of an electron with kinetic energy of 120 eV.Ans : Kinetic energy of the electron, Ek = 120 eV
Planck’s constant, h = 6.6 × 10-34 Js
Mass of an electron, m = 9.1 × 10-31 kg
Charge on an electron, e = 1.6 × 10-19 C
(a) For the electron, we can write the relation for kinetic energy as:
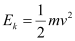
Where,
v = Speed of the electron
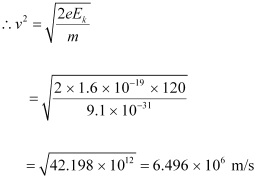
Momentum of the electron, p = mv
= 9.1 × 10-31 × 6.496 × 106
= 5.91 × 10-24 kg m s-1
Therefore, the momentum of the electron is 5.91 × 10-24 kg m s-1.
(b) Speed of the electron, v = 6.496 × 106 m/s
(c) De Broglie wavelength of an electron having a momentum p, is given as:
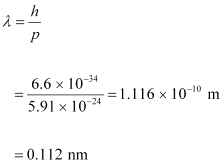
Therefore, the de Broglie wavelength of the electron is 0.112 nm.
- #13-amomentum,Ans : For the electron, we can write the relation for kinetic energy as:
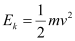
Where,
v = Speed of the electron
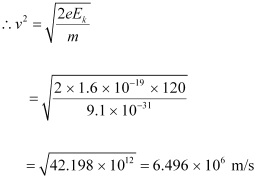
Momentum of the electron, p = mv
= 9.1 × 10-31 × 6.496 × 106
= 5.91 × 10-24 kg m s-1
Therefore, the momentum of the electron is 5.91 × 10-24 kg m s-1.
- #13-bspeed, andAns : Speed of the electron, v = 6.496 × 106 m/s
- #13-cde Broglie wavelength of an electron with kinetic energy of 120 eV.Ans : De Broglie wavelength of an electron having a momentum p, is given as:
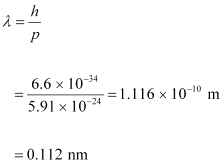
Therefore, the de Broglie wavelength of the electron is 0.112 nm.