NEET-XII-Chemistry
01: Haloalkanes and Haloarenes
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
Note: Please signup/signin free to get personalized experience.
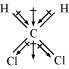
- Qstn #4-iiiCCl4Ans :
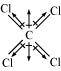
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
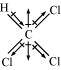
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
- Qstn #5A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans : A hydrocarbon with the molecular formula, C5H10 belongs to the group with a general molecular formula CnH2n. Therefore, it may either be an alkene or a cycloalkane.
Since hydrocarbon does not react with chlorine in the dark, it cannot be an alkene. Thus, it should be a cycloalkane.
Further, the hydrocarbon gives a single monochloro compound, C5H9Cl by reacting with chlorine in bright sunlight. Since a single monochloro compound is formed, the hydrocarbon must contain H-atoms that are all equivalent. Also, as all H-atoms of a cycloalkane are equivalent, the hydrocarbon must be a cycloalkane. Hence, the said compound is cyclopentane.
Cyclopentane (C5H10)
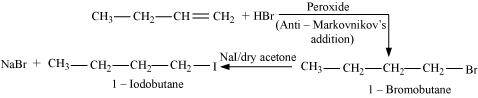
The reactions involved in the question are:
.png)
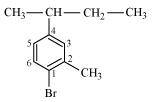
- Qstn #6Write the isomers of the compound having formula C4H9Br.
Ans : There are four isomers of the compound having the formula C4H9Br. These isomers are given below.
(a)
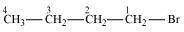
1-Bromobutane
(b)
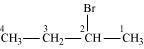
2-Bromobutane
(c)
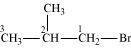
1-Bromo-2-methylpropane
(d)
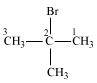
2-Bromo-2-methylpropane
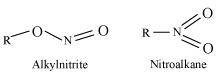
- Qstn #8What are ambident nucleophiles? Explain with an example.
Ans : Ambident nucleophiles are nucleophiles having two nucleophilic sites. Thus, ambident nucleophiles have two sites through which they can attack.
For example, nitrite ion is an ambident nucleophile.
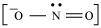
Nitrite ion can attack through oxygen resulting in the formation of alkyl nitrites. Also, it can attack through nitrogen resulting in the formation of nitroalkanes.
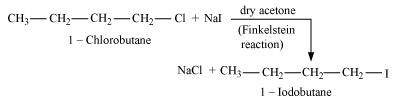
- Qstn #9-iCH3Br or CH3IAns : In the SN2 mechanism, the reactivity of halides for the same alkyl group increases in the order. This happens because as the size increases, the halide ion becomes a better leaving group.
R-F << R-Cl < R-Br < R-I
Therefore, CH3I will react faster than CH3Br in SN2 reactions with OH-.