NEET-XII-Chemistry
01: Haloalkanes and Haloarenes
- #4Which one of the following has the highest dipole moment?
() CH2Cl2
() CHCl3
() CCl4
() CHCl3
() CCl4
() CH2Cl2
() CHCl3
() CCl4
() CHCl3
() CCl4Ans : null ()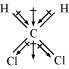
Dichlormethane (CH2Cl2)
μ = 1.60D
()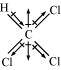
Chloroform (CHCl3)
μ = 1.08D
()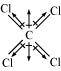
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
()
Chloroform (CHCl3)
μ = 1.08D
()
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
()
Dichlormethane (CH2Cl2)
μ = 1.60D
()
Chloroform (CHCl3)
μ = 1.08D
()
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
()
Chloroform (CHCl3)
μ = 1.08D
()
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
- #4-iCH2Cl2
() CHCl3
() CCl4Ans :
Dichlormethane (CH2Cl2)
μ = 1.60D
()
Chloroform (CHCl3)
μ = 1.08D
()
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
- #4-iiCHCl3Ans :

Chloroform (CHCl3)
μ = 1.08D
- #4-iiiCCl4Ans :

Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C-Cl bonds is opposed by the resultant of dipole moments of one C-H bond and one C-Cl bond. Since the resultant of one C-H bond and one C-Cl bond dipole moments is smaller than two C-Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in case of CH2Cl2, the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2