NEET-XII-Chemistry
02: Alcohols, Phenols and Ethers
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
02-Alcohols, Phenols and Ethers
Note: Please signup/signin free to get personalized experience.
- Qstn #11Write the mechanism of hydration of ethene to yield ethanol.
Ans : The mechanism of hydration of ethene to form ethanol involves three steps.
Step 1:
Protonation of ethene to form carbocation by electrophilic attack of H3O+:
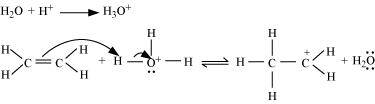
Step 2:
Nucleophilic attack of water on carbocation:
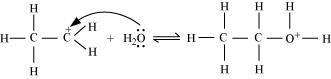
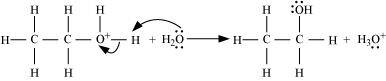
Step 3:
Deprotonation to form ethanol:
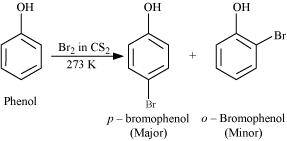
- Qstn #12You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Ans :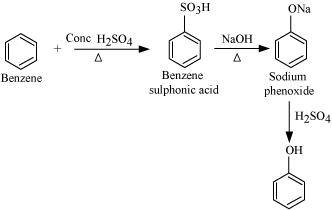
SECTION I SECTION I Page No 345:
- Qstn #13-i1-phenylethanol from a suitable alkene.Ans : By acid-catalyzed hydration of ethylbenzene (styrene), 1-phenylethanol can be synthesized.

- Qstn #13-iicyclohexylmethanol using an alkyl halide by an SN2 reaction.Ans : When chloromethylcyclohexane is treated with sodium hydroxide, cyclohexylmethanol is obtained.
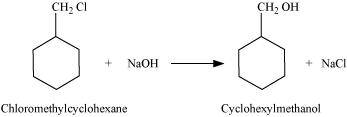
- Qstn #13-iiipentan-1-ol using a suitable alkyl halide?Ans : When 1-chloropentane is treated with NaOH, pentan-1-ol is produced.

- Qstn #14Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Ans : The acidic nature of phenol can be represented by the following two reactions:
(i) Phenol reacts with sodium to give sodium phenoxide, liberating H2.
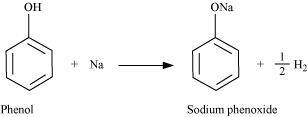
(ii) Phenol reacts with sodium hydroxide to give sodium phenoxide and water as by-products.
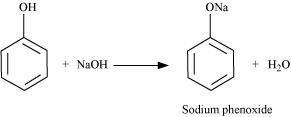
The acidity of phenol is more than that of ethanol. This is because after losing a proton, the phenoxide ion undergoes resonance and gets stabilized whereas ethoxide ion does not.
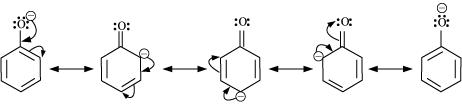
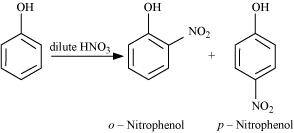
- Qstn #15Explain why is ortho nitrophenol more acidic than ortho methoxyphenol?
Ans :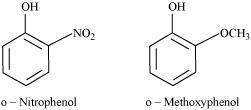
The nitro-group is an electron-withdrawing group. The presence of this group in the ortho position decreases the electron density in the O-H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid.
On the other hand, methoxy group is an electron-releasing group. Thus, it increases the electron density in the O-H bond and hence, the proton cannot be given out easily.
For this reason, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
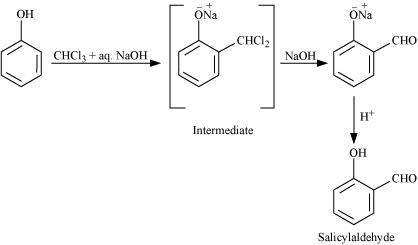
- Qstn #16Explain how does the -OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Ans : The -OH group is an electron-donating group. Thus, it increases the electron density in the benzene ring as shown in the given resonance structure of phenol.
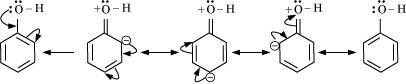
As a result, the benzene ring is activated towards electrophilic substitution.