NEET-XII-Chemistry
02: Alcohols, Phenols and Ethers
- #16Explain how does the -OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Ans : The -OH group is an electron-donating group. Thus, it increases the electron density in the benzene ring as shown in the given resonance structure of phenol.
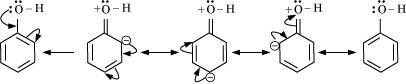
As a result, the benzene ring is activated towards electrophilic substitution.