NEET-XII-Chemistry
02: Alcohols, Phenols and Ethers
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
02-Alcohols, Phenols and Ethers
Note: Please signup/signin free to get personalized experience.
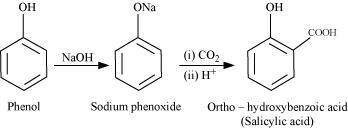
- Qstn #18-iKolbe’s reaction.Ans : Kolbe’s reaction:
When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium phenoxide when treated with carbon dioxide, followed by acidification, undergoes electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This reaction is known as Kolbe’s reaction.
- Qstn #18-iiReimer-Tiemann reaction.Ans : Reimer-Tiemann reaction:
When phenol is treated with chloroform (CHCl3) in the presence of sodium hydroxide, a -CHO group is introduced at the ortho position of the benzene ring.
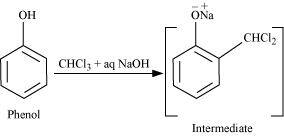
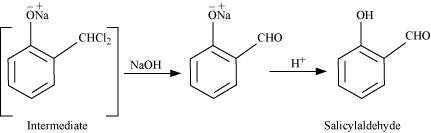
This reaction is known as the Reimer-Tiemann reaction.
The intermediate is hydrolyzed in the presence of alkalis to produce salicyclaldehyde.
- Qstn #18-iiiWilliamson ether synthesis.Ans : Williamson ether synthesis:
Williamson ether synthesis is a laboratory method to prepare symmetrical and unsymmetrical ethers by allowing alkyl halides to react with sodium alkoxides.
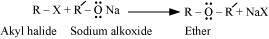
This reaction involves SN2 attack of the alkoxide ion on the alkyl halide. Better results are obtained in case of primary alkyl halides.
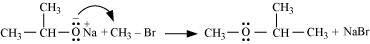
If the alkyl halide is secondary or tertiary, then elimination competes over substitution.
- Qstn #18-ivUnsymmetrical ether.Ans : Unsymmetrical ether:
An unsymmetrical ether is an ether where two groups on the two sides of an oxygen atom differ (i.e., have an unequal number of carbon atoms). For example: ethyl methyl ether (CH3-O-CH2CH3).
- Qstn #19Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
Ans : The mechanism of acid dehydration of ethanol to yield ethene involves the following three steps:
Step 1:
Protonation of ethanol to form ethyl oxonium ion:
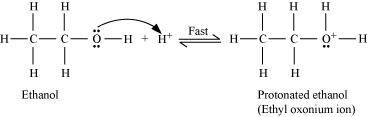
Step 2:
Formation of carbocation (rate determining step):
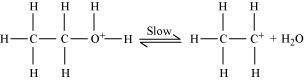
Step 3:
Elimination of a proton to form ethene:
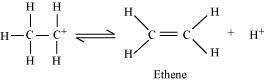
The acid consumed in step 1 is released in Step 3. After the formation of ethene, it is removed to shift the equilibrium in a forward direction.
- Qstn #20-iPropene → Propan-2-olAns : If propene is allowed to react with water in the presence of an acid as a catalyst, then propan-2-ol is obtained.
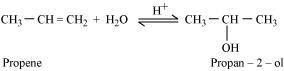
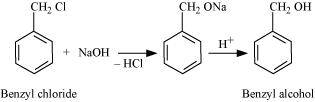
- Qstn #20-iiBenzyl chloride → Benzyl alcoholAns : If benzyl chloride is treated with NaOH (followed by acidification) then benzyl alcohol is produced.
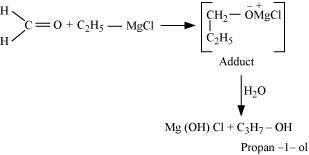
- Qstn #20-iiiEthyl magnesium chloride → Propan-1-ol.Ans : When ethyl magnesium chloride is treated with methanal, an adduct is the produced which gives propan-1-ol on hydrolysis.
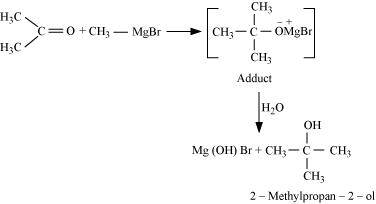
- Qstn #20-ivMethyl magnesium bromide → 2-Methylpropan-2-ol.Ans : When methyl magnesium bromide is treated with propane, an adduct is the product which gives 2-methylpropane-2-ol on hydrolysis.
- Qstn #21-iOxidation of a primary alcohol to carboxylic acid.
() Oxidation of a primary alcohol to aldehyde.
() Bromination of phenol to 2,4,6-tribromophenol.
() Benzyl alcohol to benzoic acid.
() Dehydration of propan-2-ol to propene.
() Butan-2-one to butan-2-ol.Ans : Acidified potassium permanganate
() Pyridinium chlorochromate (PCC)
() Bromine water
() Acidified potassium permanganate
() 85% phosphoric acid
() NaBH4 or LiAlH4