Loading…
NEET-XII-Chemistry
09: Coordination Compounds
- #10The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacyanoion contains only one unpaired electron. Explain using Crystal Field Theory.
Ans :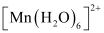
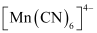
Mn is in the +2 oxidation state. Mn is in the +2 oxidation state. The electronic configuration is d5. The electronic configuration is d5. The crystal field is octahedral. Water is a weak field ligand. Therefore, the arrangement of the electrons in 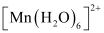 is t2g3eg2.
is t2g3eg2.The crystal field is octahedral. Cyanide is a strong field ligand. Therefore, the arrangement of the electrons in 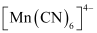 isT2g5eg0.
isT2g5eg0.
Hence, hexaaquo manganese (II) ion has five unpaired electrons, while hexacyano ion has only one unpaired electron.