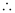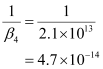Loading…
NEET-XII-Chemistry
09: Coordination Compounds
- #11Calculate the overall complex dissociation equilibrium constant for the Cu(NH3)42+ ion, given that β4 for this complex is 2.1 × 1013.
Ans : β4 = 2.1 × 1013
The overall complex dissociation equilibrium constant is the reciprocal of the overall stability constant, β4.