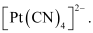NEET-XII-Chemistry
09: Coordination Compounds
- #9Predict the number of unpaired electrons in the square planar [Pt(CN)4]2- ion.
Ans :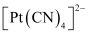
In this complex, Pt is in the +2 state. It forms a square planar structure. This means that it undergoes dsp2 hybridization. Now, the electronic configuration of Pd(+2) is 5d8.

CN- being a strong field ligand causes the pairing of unpaired electrons. Hence, there are no unpaired electrons in