NEET-XI-Chemistry
06: Hydrocarbons
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- #Chapter 6 - Hydrocarbons
- Qstn #1How do you account for the formation of ethane during chlorination of methane?
Ans : Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the given three steps.
Step 1: Initiation:
The reaction begins with the homolytic cleavage of Cl - Cl bond as:
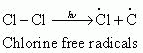
Step 2: Propagation:
In the second step, chlorine free radicals attack methane molecules and break down the C-H bond to generate methyl radicals as:
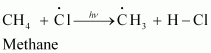
These methyl radicals react with other chlorine free radicals to form methyl chloride along with the liberation of a chlorine free radical.
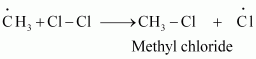
Hence, methyl free radicals and chlorine free radicals set up a chain reaction. While HCl and CH3Cl are the major products formed, other higher halogenated compounds are also formed as:
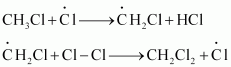
Step 3: Termination:
Formation of ethane is a result of the termination of chain reactions taking place as a result of the consumption of reactants as:
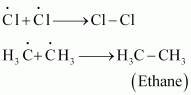
Hence, by this process, ethane is obtained as a by-product of chlorination of methane.
- #2-a
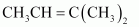 Ans :
Ans :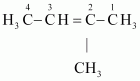
IUPAC name: 2-Methylbut-2-ene
- #2-b
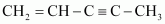 Ans :
Ans :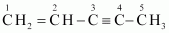
IUPAC name: Pen-1-ene-3-yne
- #2-c
 Ans :
Ans : can be written as:
can be written as:
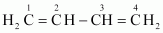
IUPAC name: 1, 3-Butadiene or Buta-1,3-diene
- #2-d
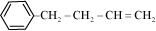 Ans :
Ans :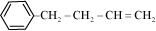
IUPAC name: 4-Phenyl but-1-ene
- #2-e
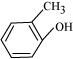 Ans :
Ans :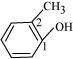
IUPAC name: 2-Methyl phenol
- #2-f
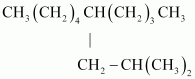
Ans :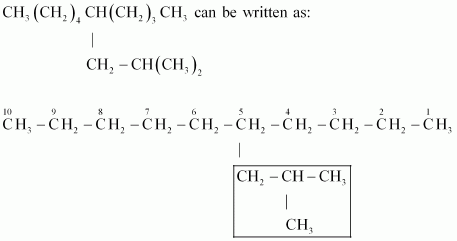
IUPAC name: 5-(2-Methylpropyl)-decane
- #2-g
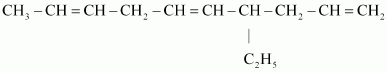
Ans :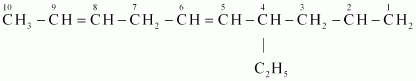
IUPAC name: 4-Ethyldeca-1, 5, 8-triene
- Qstn #3For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
- #3-aC4H8 (one double bond)Ans : The following structural isomers are possible for C4H8 with one double bond:
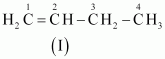
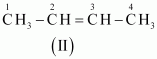
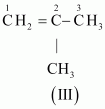
The IUPAC name of
Compound (I) is But-1-ene,
Compound (II) is But-2-ene, and
Compound (III) is 2-Methylprop-1-ene.
- #3-bC5H8 (one triple bond)Ans : The following structural isomers are possible for C5C8 with one triple bond:
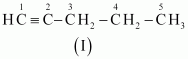
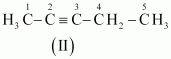
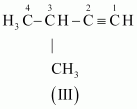
The IUPAC name of
Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-yne.
- Qstn #4-iPent-2-eneAns : Pent-2-ene undergoes ozonolysis as:
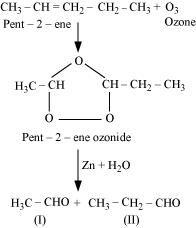
The IUPAC name of Product (I) is ethanal and Product (II)is propanal.