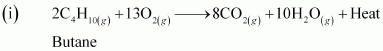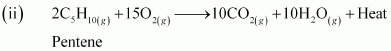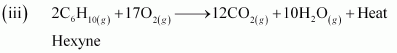NEET-XI-Chemistry
06: Hydrocarbons
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Qstn #4-ii3,4-Dimethyl-hept-3-eneAns : 3, 4-Dimethylhept-3-ene undergoes ozonolysis as:
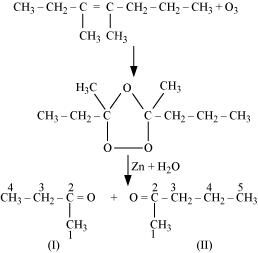
The IUPAC name of Product (I)is butan-2-one and Product (II)is Pentan-2-one.
- Qstn #4-iii2-Ethylbut-1-eneAns : 2-Ethylbut-1-ene undergoes ozonolysis as:
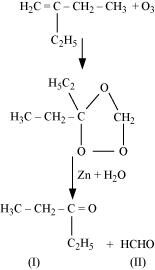
The IUPAC name of Product (I)is pentan-3-one and Product (II)is methanal.
- Qstn #4-iv1-Phenylbut-1-eneAns : 1-Phenylbut-1-ene undergoes ozonolysis as:
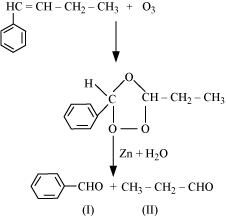
The IUPAC name of Product (I)is benzaldehyde and Product (II)is propanal.
Page No 397:
- Qstn #5An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of ‘A’.
Ans :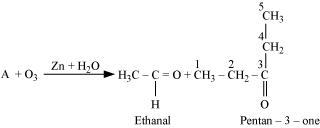
During ozonolysis, an ozonide having a cyclic structure is formed as an intermediate which undergoes cleavage to give the final products. Ethanal and pentan-3-one are obtained from the intermediate ozonide. Hence, the expected structure of the ozonide is:
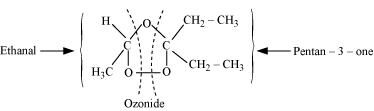
This ozonide is formed as an addition of ozone to ‘A’. The desired structure of ‘A’ can be obtained by the removal of ozone from the ozonide. Hence, the structural formula of ‘A’ is:
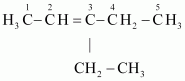
The IUPAC name of ‘A’ is 3-Ethylpent-2-ene.
- Qstn #6An alkene ‘A’ contains three C - C, eight C - H σ bonds and one C - C ``\pi`` bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Ans : As per the given information, ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. The formation of two moles of an aldehyde indicates the presence of identical structural units on both sides of the double bond containing carbon atoms. Hence, the structure of ‘A’ can be represented as:
XC = CX
There are eight C-H σ bonds. Hence, there are 8 hydrogen atoms in ‘A’. Also, there are three C-C bonds. Hence, there are four carbon atoms present in the structure of ‘A’.
Combining the inferences, the structure of ‘A’ can be represented as:
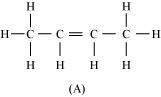
‘A’ has 3 C-C bonds, 8 C-H σ bonds, and one C-C ``\pi`` bond.
Hence, the IUPAC name of ‘A’ is But-2-ene.
Ozonolysis of ‘A’ takes place as:
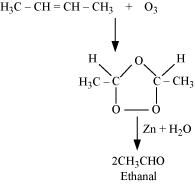
The final product is ethanal with molecular mass
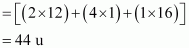
- Qstn #7Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Ans : As per the given information, propanal and pentan-3-one are the ozonolysis products of an alkene. Let the given alkene be ‘A’. Writing the reverse of the ozonolysis reaction, we get:
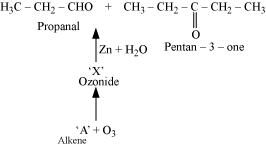
The products are obtained on the cleavage of ozonide ‘X’. Hence, ‘X’ contains both products in the cyclic form. The possible structure of ozonide can be represented as:
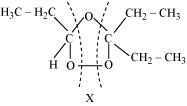
Now, ‘X’ is an addition product of alkene ‘A’ with ozone. Therefore, the possible structure of alkene ‘A’ is:
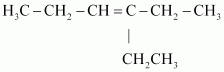
- Qstn #8Write chemical equations for combustion reaction of the following hydrocarbons:
Ans : Combustion can be defined as a reaction of a compound with oxygen.
- Qstn #9Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Ans : Hex-2-ene is represented as:
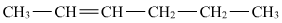
Geometrical isomers of hex-2-ene are:

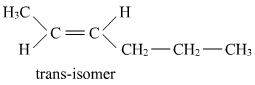
The dipole moment of cis-compound is a sum of the dipole moments of C-CH3 and C-CH2CH2CH3 bonds acting in the same direction.
The dipole moment of trans-compound is the resultant of the dipole moments of C-CH3 and C-CH2CH2CH3 bonds acting in opposite directions.
Hence, cis-isomer is more polar than trans-isomer. The higher the polarity, the greater is the intermolecular dipole-dipole interaction and the higher will be the boiling point. Hence, cis-isomer will have a higher boiling point than trans-isomer.
- Qstn #10Why is benzene extra ordinarily stable though it contains three double bonds?
Ans : Benzene is a hybrid of resonating structures given as:

All six carbon atoms in benzene are sp2 hybridized. The two sp2 hybrid orbitals of each carbon atom overlap with the sp2 hybrid orbitals of adjacent carbon atoms to form six sigma bonds in the hexagonal plane. The remaining sp2 hybrid orbital on each carbon atom overlaps with the s-orbital of hydrogen to form six sigma C-H bonds. The remaining unhybridized p-orbital of carbon atoms has the possibility of forming three ``\pi`` bonds by the lateral overlap of
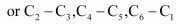 .
.
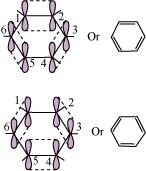
The six ``\pi``’s are delocalized and can move freely about the six carbon nuclei. Even after the presence of three double bonds, these delocalized ``\pi``-electrons stabilize benzene.
- Qstn #11What are the necessary conditions for any system to be aromatic?
Ans : A compound is said to be aromatic if it satisfies the following three conditions:
-(i) It should have a planar structure.
(ii) The ``\pi``-electrons of the compound are completely delocalized in the ring.
(iii) The total number of ``\pi``-electrons present in the ring should be equal to (4n + 2), where n = 0, 1, 2 ... etc. This is known as Huckel’s rule.