NEET-XI-Chemistry
02: Hydrogen
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Ans :
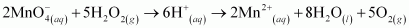
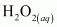 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 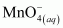 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
- Ans :
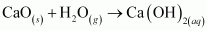
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
- Ans :
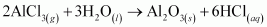
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
- Qstn #20-v

Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Ans :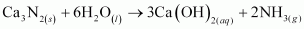
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
- Qstn #21Describe the structure of the common form of ice.
Ans : Ice is the crystalline form of water. It takes a hexagonal form if crystallized at atmospheric pressure, but condenses to cubic form if the temperature is very low.
The three-dimensional structure of ice is represented as:
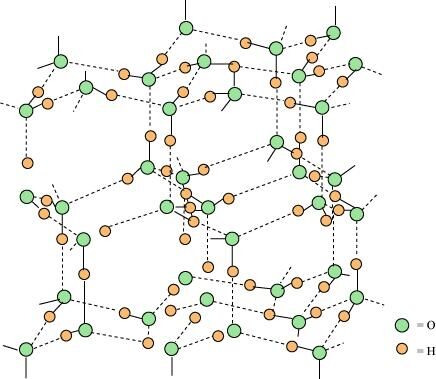
The structure is highly ordered and has hydrogen bonding. Each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm. The structure also contains wide holes that can hold molecules of appropriate sizes interstitially.
- Qstn #22What causes the temporary and permanent hardness of water?
Ans : Temporary hardness of water is due to the presence of soluble salts of magnesium and calcium in the form of hydrogen carbonates (MHCO3, where M = Mg, Ca) in water.
Permanent hardness of water is because of the presence of soluble salts of calcium and magnesium in the form of chlorides in water.
- Qstn #23Discuss the principle and method of softening of hard water by synthetic ion-exchange resins.
Ans : The process of treating permanent hardness of water using synthetic resins is based on the exchange of cations (e.g., Na+, Ca2+, Mg2+ etc) and anions (e.g., Cl-, SO42-, HCO3- etc) present in water by H+ and OH- ions respectively.
Synthetic resins are of two types:
1) Cation exchange resins
2) Anion exchange resins
Cation exchange resins are large organic molecules that contain the -SO3H group. The resin is firstly changed to RNa (from RSO3H) by treating it with NaCl. This resin then exchanges Na+ ions with Ca2+ and Mg2+ ions, thereby making the water soft.

There are cation exchange resins in H+ form. The resins exchange H+ ions for Na+, Ca2+, and Mg2+ ions.
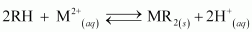
Anion exchange resins exchange OH- ions for anions like Cl-,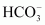 , and SO42- present in water.
, and SO42- present in water.
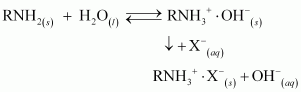
During the complete process, water first passes through the cation exchange process. The water obtained after this process is free from mineral cations and is acidic in nature.
This acidic water is then passed through the anion exchange process where OH- ions neutralize the H+ ions and de-ionize the water obtained.
- Qstn #24Write chemical reactions to show the amphoteric nature of water.
Ans : The amphoteric nature of water can be described on the basis of the following reactions:
1) Reaction with H2S
The reaction takes place as:

In the forward reaction, accepts a proton from
accepts a proton from . Hence, it acts as a Lewis base.
. Hence, it acts as a Lewis base.
2) Reaction with NH3
The reaction takes place as:

In the forward reaction, denotes its proton to
denotes its proton to . Hence, it acts as a Lewis acid.
. Hence, it acts as a Lewis acid.
3) Self-ionization of water
In the reaction, two water molecules react as:

- Qstn #25Write chemical reactions to justify that hydrogen peroxide can function as an oxidizing as well as reducing agent.
Ans : Hydrogen peroxide, H2O2 acts as an oxidizing as well as a reducing agent in both acidic and alkaline media.
Reactions involving oxidizing actions are:
1)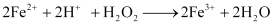
2)
3)
4)
Reactions involving reduction actions are:
1)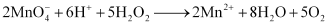
2)
3)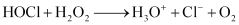
4)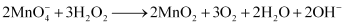
Page No 290:
- Qstn #26What is meant by ‘de-mineralised’ water and how can it be obtained?
Ans : De-mineralised water is free from all soluble mineral salts. It does not contain any anions or cations.
De-mineralised water is obtained by passing water successively through a cation exchange (in the H+ form) and an anion exchange (in the OH- form) resin.
During the cation exchange process, H+ exchanges for Na+, Mg2+, Ca2+, and other cations present in water.
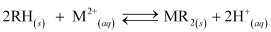 ....... (1)
....... (1)
In the anion exchange process, OH- exchanges for anions such as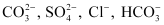 etc. present in water.
etc. present in water.
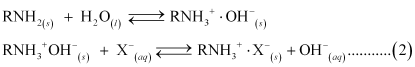
OH- ions liberated in reaction (2) neutralize H+ ions liberated in reaction (1), thereby forming water.

- Qstn #27Is demineralised or distilled water useful for drinking purposes? If not, how can it be made useful?
Ans : Water is an important part of life. It contains several dissolved nutrients that are required by human beings, plants, and animals for survival. Demineralised water is free of all soluble minerals. Hence, it is not fit for drinking.
It can be made useful only after the addition of desired minerals in specific amounts, which are important for growth.
- Qstn #28Describe the usefulness of water in biosphere and biological systems.
Ans : Water is essential for all forms of life. It constitutes around 65% of the human body and 95% of plants. Water plays an important role in the biosphere owing to its high specific heat, thermal conductivity, surface tension, dipole moment, and dielectric constant.
The high heat of vapourization and heat of capacity of water helps in moderating the climate and body temperature of all living beings.
It acts as a carrier of various nutrients required by plants and animals for various metabolic reactions.
- Qstn #29What properties of water make it useful as a solvent? What types of compound can it (i) dissolve, and (ii) hydrolyse?
Ans : A high value of dielectric constants (78.39 C2/Nm2) and dipole moment make water a universal solvent.
Water is able to dissolve most ionic and covalent compounds. Ionic compounds dissolve in water because of the ion-dipole interaction, whereas covalent compounds form hydrogen bonding and dissolve in water.
Water can hydrolyze metallic and non-metallic oxides, hydrides, carbides, phosphides, nitrides and various other salts. During hydrolysis, H+ and OH- ions of water interact with the reacting molecule.
Some reactions are:
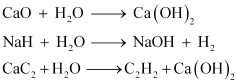
- Qstn #30Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes?
Ans : Heavy water (D2O) acts as a moderator, i.e., it slows the rate of a reaction. Due to this property of D2O, it cannot be used for drinking purposes because it will slow down anabolic and catabolic reactions taking place in the body and lead to a casualty.
- Qstn #31What is the difference between the terms ‘hydrolysis’ and ‘hydration’?
Ans : Hydrolysis is defined as a chemical reaction in which hydrogen and hydroxide ions (H+ and OH- ions) of water molecule react with a compound to form products. For example:
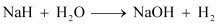
Hydration is defined as the addition of one or more water molecules to ions or molecules to form hydrated compounds. For example: