NEET-XI-Chemistry
02: Hydrogen
- #26What is meant by ‘de-mineralised’ water and how can it be obtained?
Ans : De-mineralised water is free from all soluble mineral salts. It does not contain any anions or cations.
De-mineralised water is obtained by passing water successively through a cation exchange (in the H+ form) and an anion exchange (in the OH- form) resin.
During the cation exchange process, H+ exchanges for Na+, Mg2+, Ca2+, and other cations present in water.
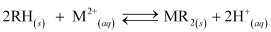 ....... (1)
....... (1)
In the anion exchange process, OH- exchanges for anions such as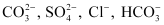 etc. present in water.
etc. present in water.
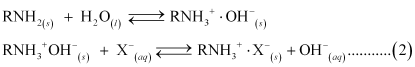
OH- ions liberated in reaction (2) neutralize H+ ions liberated in reaction (1), thereby forming water.
