NEET-XI-Chemistry
02: Hydrogen
- #20-i
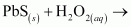
()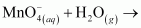
()
()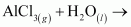
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
()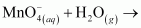
()
()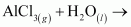
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
()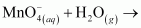
()
()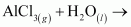
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
()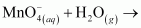
()
()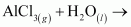
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
()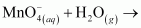
()
()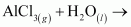
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
()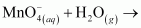
()
()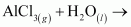
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Ans :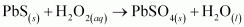
H2O2 is acting as an oxidizing agent in the reaction. Hence, it is a redox reaction.
()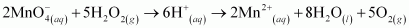
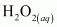 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 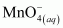 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()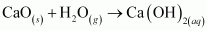
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()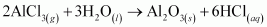
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()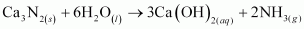
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
()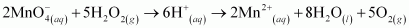
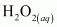 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 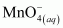 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()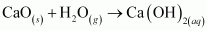
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()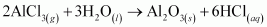
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()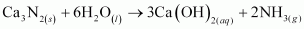
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
()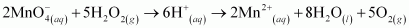
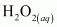 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 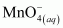 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()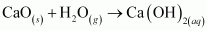
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()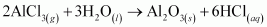
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()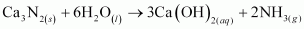
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
()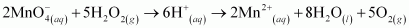
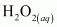 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 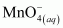 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()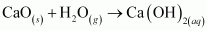
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()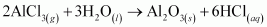
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()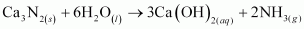
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
()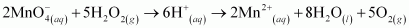
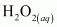 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 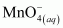 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()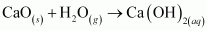
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()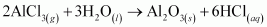
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()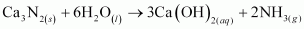
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
()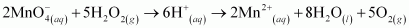
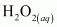 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 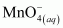 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()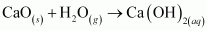
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()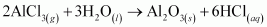
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()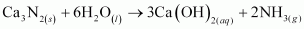
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
- #20-ii
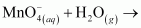 Ans :
Ans :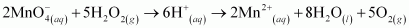
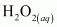 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 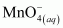 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
- #20-iii
 Ans :
Ans :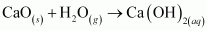
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
- #20-iv
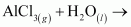 Ans :
Ans :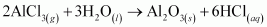
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
- #20-v

Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Ans :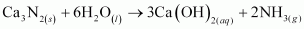
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.