NEET-XI-Chemistry
02: Hydrogen
Note: Please signup/signin free to get personalized experience.
Note: Please signup/signin free to get personalized experience.
10 minutes can boost your percentage by 10%
No item to list.
Note: Please signup/signin free to get personalized experience.
- Qstn #11What do you understand by the term “non-stoichiometric hydrides”? Do you expect this type of the hydrides to be formed by alkali metals? Justify your answer.
Ans : Non-Stoichiometric hydrides are hydrogen-deficient compounds formed by the reaction of dihydrogen with d-block and f-block elements. These hydrides do not follow the law of constant composition. For example: LaH2.87, YbH2.55, TiH1.5 - 1.8 etc.
Alkali metals form stoichiometric hydrides. These hydrides are ionic in nature. Hydride ions have comparable sizes (208 pm) with alkali metal ions. Hence, strong binding forces exist between the constituting metal and hydride ion. As a result, stoichiometric hydrides are formed.
Alkali metals will not form non-stoichiometric hydrides.
- Qstn #12How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Ans : Metallic hydrides are hydrogen deficient, i.e., they do not hold the law of constant composition. It has been established that in the hydrides of Ni, Pd, Ce, and Ac, hydrogen occupies the interstitial position in lattices allowing further absorption of hydrogen on these metals. Metals like Pd, Pt, etc. have the capacity to accommodate a large volume of hydrogen. Therefore, they are used for the storage of hydrogen and serve as a source of energy.
- Qstn #13How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain.
Ans : Atomic hydrogen atoms are produced by the dissociation of dihydrogen with the help of an electric arc. This releases a huge amount of energy (435.88 kJ mol-1). This energy can be used to generate a temperature of 4000 K, which is ideal for welding and cutting metals. Hence, atomic hydrogen or oxy-hydrogen torches are used for these purposes. For this reason, atomic hydrogen is allowed to recombine on the surface to be welded to generate the desired temperature.
- Qstn #14Among NH3, H2O and HF, which would you expect to have highest magnitude of hydrogen bonding and why?
Ans : The extent of hydrogen bonding depends upon electronegativity and the number of hydrogen atoms available for bonding. Among nitrogen, fluorine, and oxygen, the increasing order of their electronegativities are N < O < F.
Hence, the expected order of the extent of hydrogen bonding is HF > H2O > NH3.
But, the actual order is H2O > HF > NH3.
Although fluorine is more electronegative than oxygen, the extent of hydrogen bonding is higher in water. There is a shortage of hydrogens in HF, whereas there are exactly the right numbers of hydrogens in water. As a result, only straight chain bonding takes place. On the other hand, oxygen forms a huge ring-like structure through its high ability of hydrogen bonding.
In case of ammonia, the extent of hydrogen bonding is limited because nitrogen has only one lone pair. Therefore, it cannot satisfy all hydrogens.
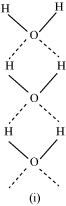
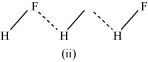
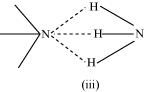
- Qstn #15Saline hydrides are known to react with water violently producing fire. Can CO2, a well known fire extinguisher, be used in this case? Explain.
Ans : Saline hydrides (i.e., NaH, LiH, etc.) react with water to form a base and hydrogen gas. The chemical equation used to represent the reaction can be written as:

The reaction is violent and produces fire.
CO2 is heavier than dioxygen. It is used as a fire extinguisher because it covers the fire as a blanket and inhibits the supply of dioxygen, thereby dousing the fire.
CO2 can be used in the present case as well. It is heavier than dihydrogen and will be effective in isolating the burning surface from dihydrogen and dioxygen.
- Qstn #16-iCaH2, BeH2 and TiH2 in order of increasing electrical conductance.Ans : The electrical conductance of a molecule depends upon its ionic or covalent nature. Ionic compounds conduct, whereas covalent compounds do not.
BeH2 is a covalent hydride. Hence, it does not conduct. CaH2 is an ionic hydride, which conducts electricity in the molten state. Titanium hydride, TiH2 is metallic in nature and conducts electricity at room temperature. Hence, the increasing order of electrical conductance is as follows:
BeH2 < CaH2 < TiH2
- Qstn #16-iiLiH, NaH and CsH in order of increasing ionic character.Ans : The ionic character of a bond is dependent on the electronegativities of the atoms involved. The higher the difference between the electronegativities of atoms, the smaller is the ionic character.
Electronegativity decreases down the group from Lithium to Caesium. Hence, the ionic character of their hydrides will increase (as shown below).
LiH < NaH < CsH
- Qstn #16-iiiH-H, D-D and F-F in order of increasing bond dissociation enthalpy.Ans : Bond dissociation energy depends upon the bond strength of a molecule, which in turn depends upon the attractive and repulsive forces present in a molecule.
The bond pair in D-D bond is more strongly attracted by the nucleus than the bond pair in H-H bond. This is because of the higher nuclear mass of D2. The stronger the attraction, the greater will be the bond strength and the higher is the bond dissociation enthalpy. Hence, the bond dissociation enthalpy of D-D is higher than H-H.
However, bond dissociation enthalpy is the minimum in the case of F-F. The bond pair experiences strong repulsion from the lone pairs present on each F-centre.
Therefore, the increasing order of bond dissociation enthalpy is as follows:
F-F < H-H < D-D
- Qstn #16-ivNaH, MgH2 and H2O in order of increasing reducing property.Ans : Ionic hydrides are strong reducing agents. NaH can easily donate its electrons. Hence, it is most reducing in nature.
Both, MgH2 and H2O are covalent hydrides. H2O is less reducing than MgH2 since the bond dissociation energy of H2O is higher than MgH2.
Hence, the increasing order of the reducing property is H2O < MgH2 < NaH.
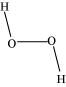
- Qstn #17Compare the structures of H2O and H2O2.
Ans : In gaseous phase, water molecule has a bent form with a bond angle of 104.5°. The O-H bond length is 95.7 pm. The structure can be shown as:
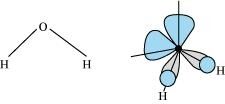
Hydrogen peroxide has a non-planar structure both in gas and solid phase. The dihedral angle in gas and solid phase is 111.5° and 90.2° respectively.
- Qstn #18What do you understand by the term ’auto-protolysis’ of water? What is its significance?
Ans : Auto-protolysis (self-ionization) of water is a chemical reaction in which two water molecules react to produce a hydroxide ion (OH-) and a hydronium ion (H3O+).
The reaction involved can be represented as:

Auto-protolysis of water indicates its amphoteric nature i.e., its ability to act as an acid as well as a base.
The acid-base reaction can be written as:

- Qstn #19Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidized/reduced.
Ans : The reaction between fluorine and water can be represented as:
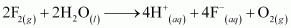
This is an example of a redox reaction as water is getting oxidized to oxygen, while fluorine is being reduced to fluoride ion.
The oxidation numbers of various species can be represented as:
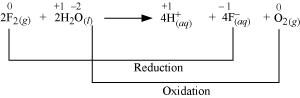
Fluorine is reduced from zero to (- 1) oxidation state. A decrease in oxidation state indicates the reduction of fluorine.
Water is oxidized from (- 2) to zero oxidation state. An increase in oxidation state indicates oxidation of water.
- Ans :
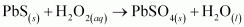
H2O2 is acting as an oxidizing agent in the reaction. Hence, it is a redox reaction.