NEET-XI-Chemistry
02: Hydrogen
- #20-i
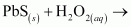
()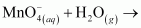
()
()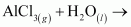
()
Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Ans :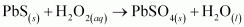
H2O2 is acting as an oxidizing agent in the reaction. Hence, it is a redox reaction.
()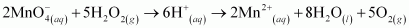
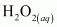 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 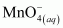 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
()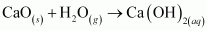
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
()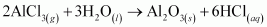
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
()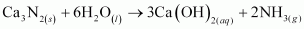
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.
- #20-ii
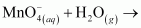 Ans :
Ans :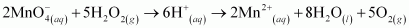
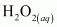 is acting as a reducing agent in the acidic medium, thereby oxidizing
is acting as a reducing agent in the acidic medium, thereby oxidizing 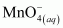 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
- #20-iii
 Ans :
Ans :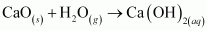
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction is hydrolysis.
- #20-iv
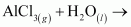 Ans :
Ans :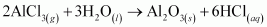
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of AlCl3.
- #20-v

Classify the above into (a) hydrolysis, (b) redox and (c) hydration reactions.
Ans :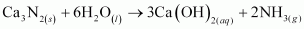
The reactions in which a compound reacts with water to produce other compounds are called hydrolysis reactions. The given reaction represents hydrolysis of Ca3N2.