NEET-XI-Chemistry
05: States of Matter
- #16Pay load is defined as the difference between the mass of displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10 m, mass 100 kg is filled with helium at 1.66 bar at 27°C. (Density of air = 1.2 kg m-3 and R = 0.083 bar dm3 K-1 mol-1).
Ans : Given,
Radius of the balloon, r = 10 m
.gif) Volume of the balloon
Volume of the balloon 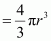
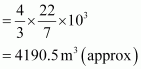
Thus, the volume of the displaced air is 4190.5 m3.
Given,
Density of air = 1.2 kg m-3
Then, mass of displaced air = 4190.5 × 1.2 kg
= 5028.6 kg
Now, mass of helium (m) inside the balloon is given by,
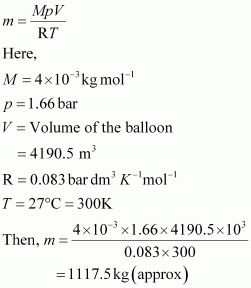
Now, total mass of the balloon filled with helium = (100 + 1117.5) kg
= 1217.5 kg
Hence, pay load = (5028.6 - 1217.5) kg
= 3811.1 kg
Hence, the pay load of the balloon is 3811.1 kg.